Topiramate
These highlights do not include all the information needed to use TOPIRAMATE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for TOPIRAMATE EXTENDED-RELEASE CAPSULES. TOPIRAMATE EXTENDED-RELEASE capsules, for oral use Initial U.S. Approval: 1996
691d276c-9cc1-4d39-828f-0f44d7ef6328
HUMAN PRESCRIPTION DRUG LABEL
Jun 24, 2025
Upsher-Smith Laboratories, LLC
DUNS: 047251004
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Topiramate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Topiramate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Topiramate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Topiramate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Topiramate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
NDC 0832-1073-30
Once-Daily Dosing
Topiramate
Extended-Release
Capsules
200 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
30 Capsules
Rx only

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Monotherapy Epilepsy
Topiramate extended-release capsules are indicated as initial monotherapy for the treatment of partial-onset or primary generalized tonic-clonic seizures in patients 2 years of age and older.
1.2 Adjunctive Therapy Epilepsy
Topiramate extended-release capsules are indicated as adjunctive therapy for the treatment of partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut Syndrome in patients 2 years of age and older.
1.3 Migraine
Topiramate extended-release capsules are indicated for the preventive treatment of migraine in patients 12 years of age and older.
Topiramate extended-release capsules are indicated for: (1)
- Epilepsy: initial monotherapy for the treatment of partial-onset or primary generalized tonic-clonic seizures in patients 2 years of age and older ( 1.1); adjunctive therapy for the treatment of partial-onset seizures, primary generalized tonic-clonic seizures, or seizures associated with Lennox-Gastaut Syndrome in patients 2 years of age and older ( 1.2)
- Preventive treatment of migraine in patients 12 years of age and older ( 1.3)
DESCRIPTION SECTION
11 DESCRIPTION
Topiramate, USP, is a sulfamate-substituted monosaccharide. Topiramate extended-release capsules are available as 25 mg, 50 mg, 100 mg, 150 mg, and 200 mg capsules for oral administration as whole capsules or opened and sprinkled onto a spoonful of soft food.
Topiramate is a white to off-white powder. Topiramate is freely soluble in polar organic solvents such as acetonitrile and acetone; and very slightly soluble to practically insoluble in non-polar organic solvents such as hexanes. Topiramate has the molecular formula C 12H 21NO 8S and a molecular weight of 339.4. Topiramate is designated chemically as 2,3:4,5-Di-O- isopropylidene-β-D-fructopyranose sulfamate and has the following structural formula:
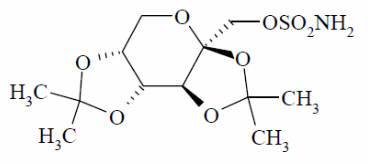
Topiramate extended-release capsules contain beads of topiramate in a capsule. The inactive ingredients are microcrystalline cellulose, hypromellose 2910, ethylcellulose, diethyl phthalate.
In addition, the capsule shells for all strengths contain hypromellose 2910, titanium dioxide, black iron oxide, red iron oxide and/or yellow iron oxide, black pharmaceutical ink, and white pharmaceutical ink (200 mg only).
Meets USP Dissolution Test 2
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanisms by which topiramate exerts its anticonvulsant and preventive migraine effects are unknown; however, preclinical studies have revealed four properties that may contribute to topiramate’s efficacy for epilepsy and the preventive treatment of migraine. Electrophysiological and biochemical evidence suggests that topiramate, at pharmacologically relevant concentrations, blocks voltage-dependent sodium channels, augments the activity of the neurotransmitter gamma-aminobutyrate at some subtypes of the GABA‑A receptor, antagonizes the AMPA/kainate subtype of the glutamate receptor, and inhibits the carbonic anhydrase enzyme, particularly isozymes II and IV.
12.2 Pharmacodynamics
Topiramate has anticonvulsant activity in rat and mouse maximal electroshock seizure (MES) tests. Topiramate is only weakly effective in blocking clonic seizures induced by the GABA-A receptor antagonist, pentylenetetrazole. Topiramate is also effective in rodent models of epilepsy, which include tonic and absence-like seizures in the spontaneous epileptic rat (SER) and tonic and clonic seizures induced in rats by kindling of the amygdala or by global ischemia.
Changes (increases and decreases) from baseline in vital signs (systolic blood pressure-SBP, diastolic blood pressure-DBP, pulse) occurred more frequently in pediatric patients (6 to 17 years) treated with various daily doses of topiramate (50 mg, 100 mg, 200 mg, 2 to 3 mg/kg) than in patients treated with placebo in controlled trials for the preventive treatment of migraine. The most notable changes were SBP < 90 mm Hg, DBP < 50 mm Hg, SBP or DBP increases or decreases ≥ 20 mm Hg, and pulse increases or decreases ≥ 30 beats per minute. These changes were often dose-related and were most frequently associated with the greatest treatment difference at the 200 mg dose level. Systematic collection of orthostatic vital signs has not been conducted. The clinical significance of these various changes in vital signs has not been clearly established.
12.3 Pharmacokinetics
Absorption and Distribution
The pharmacokinetics of topiramate extended-release are linear with dose proportional increases in plasma concentration when administered as a single oral dose over the range of 50 mg to 1,400 mg. At 25 mg, the pharmacokinetics of topiramate extended-release are nonlinear, possibly due to the binding of topiramate to carbonic anhydrase in red blood cells.
Topiramate extended-release capsules sprinkled on a spoonful of soft food is bioequivalent to the intact capsule formulation.
Following a single 200 mg oral dose of topiramate extended-release, peak plasma concentrations (Tmax) occurred approximately 20 hours after dosing. Steady-state was reached in about 5 days following daily dosing of topiramate extended-release in subjects with normal renal function, with a Tmax of approximately 6 hours.
At steady-state, the plasma exposure (AUC0–24hr, Cmax, and Cmin) of topiramate from topiramate extended-release administered once daily and the immediate- release topiramate tablets administered twice‑daily were shown to be bioequivalent. Fluctuation of topiramate plasma concentrations at steady-state for topiramate extended-release administered once daily was approximately 40% in healthy subjects, compared to approximately 53% for immediate-release topiramate [see Clinical Pharmacology (12.6)].
Compared to the fasted state, high-fat meal had no effect on bioavailability (AUC and Cmax) but delayed the Tmax by approximately 4 hours following a single dose of topiramate extended-release. Topiramate extended-release capsules can be taken without regard to meals.
Topiramate is 15% to 41% bound to human plasma proteins over the blood concentration range of 0.5 mcg/mL to 250 mcg/mL. The fraction bound decreased as blood concentration increased.
Carbamazepine and phenytoin do not alter the binding of immediate-release topiramate. Sodium valproate, at 500 mcg/mL (a concentration 5 to 10 times higher than considered therapeutic for valproate) decreased the protein binding of immediate-release topiramate from 23% to 13%. Immediate-release topiramate does not influence the binding of sodium valproate.
Metabolism and Excretion
Topiramate is not extensively metabolized and is primarily eliminated unchanged in the urine (approximately 70% of an administered dose). Six metabolites have been identified in humans, none of which constitutes more than 5% of an administered dose. The metabolites are formed via hydroxylation, hydrolysis, and glucuronidation. There is evidence of renal tubular reabsorption of topiramate. In rats, given probenecid to inhibit tubular reabsorption, along with topiramate, a significant increase in renal clearance of topiramate was observed. This interaction has not been evaluated in humans. Overall, oral plasma clearance (CL/F) is approximately 20 mL/min to 30 mL/min in adults following oral administration. The mean effective half-life of topiramate extended-release is approximately 56 hours. Steady-state is reached in about 5 days after topiramate extended-release dosing in subjects with normal renal function.
Specific Populations
Renal Impairment
The clearance of topiramate was reduced by 42% in subjects with moderate renal impairment (creatinine clearance 30 to 69 mL/min/1.73 m 2) and by 54% in subjects with severe renal impairment (creatinine clearance less than 30 mL/min/1.73 m 2) compared to subjects with normal renal function (creatinine clearance greater than 70 mL/min/1.73 m 2) [see Dosage and Administration (2.4, 2.6)] .
Hemodialysis
Topiramate is cleared by hemodialysis. Using a high-efficiency, counter flow, single pass-dialysate hemodialysis procedure, topiramate dialysis clearance was 120 mL/min with blood flow through the dialyzer at 400 mL/min. This high clearance (compared to 20 mL/min to 30 mL/min total oral clearance in healthy adults) will remove a clinically significant amount of topiramate from the patient over the hemodialysis treatment period [see Dosage and Administration (2.5)and Use in Specific Populations (8.7)] .
Hepatic Impairment
Plasma clearance of topiramate decreased a mean of 26% in patients with moderate to severe hepatic impairment.
Age, Gender and Race
The pharmacokinetics of topiramate in elderly subjects (65 to 85 years of age, N=16) were evaluated in a controlled clinical study. The elderly subject population had reduced renal function (creatinine clearance [-20%]) compared to young adults. Following a single oral 100 mg dose, maximum plasma concentration for elderly and young adults was achieved at approximately 1 to 2 hours. Reflecting the primary renal elimination of topiramate, topiramate plasma and renal clearance were reduced 21% and 19%, respectively, in elderly subjects, compared to young adults. Similarly, topiramate half-life was longer (13%) in the elderly. Reduced topiramate clearance resulted in slightly higher maximum plasma concentration (23%) and AUC (25%) in elderly subjects than observed in young adults. Topiramate clearance is decreased in the elderly only to the extent that renal function is reduced [see Dosage and Administration (2.3), Use in Specific Populations (8.5)] .
Clearance of topiramate in adults was not affected by gender or race.
Pediatric Pharmacokinetics
Pharmacokinetics of immediate-release topiramate were evaluated in patients age 2 years to less than 16 years. Patients received either no or a combination of other antiepileptic drugs. A population pharmacokinetic model was developed on the basis of pharmacokinetic data from relevant topiramate clinical studies. This dataset contained data from 1,217 subjects including 258 pediatric patients age 2 years to less than 16 years (95 pediatric patients less than 10 years of age).
Pediatric patients on adjunctive treatment exhibited a higher oral clearance (L/h) of topiramate compared to patients on monotherapy, presumably because of increased clearance from concomitant enzyme-inducing antiepileptic drugs. In comparison, topiramate clearance per kg is greater in pediatric patients than in adults and in young pediatric patients (down to 2 years) than in older pediatric patients. Consequently, the plasma drug concentration for the same mg/kg/day dose would be lower in pediatric patients compared to adults and also in younger pediatric patients compared to older pediatric patients. Clearance was independent of dose.
As in adults, hepatic enzyme-inducing antiepileptic drugs decrease the steady state plasma concentrations of topiramate.
Pediatric Patients with Obesity
A population PK analysis of topiramate was conducted in 129 children <21 years of age with or without obesity to evaluate the potential impact of obesity on plasma topiramate exposures. Obesity was defined as BMI ≥ 95th percentile for age and sex based on CDC-recommended BMI-for-age growth charts for males and females. Using the currently recommended dosing regimens, children with obesity are likely to have median values of average concentration at steady- state, and trough concentration at steady-state up to 20% lower and 19% lower, respectively, compared to children without obesity. Dosage adjustment according to obesity status is not necessary.
Drug Interactions
In vitrostudies indicate that topiramate does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, or CYP3A4/5 isozymes. In vitrostudies indicate that topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4.
Antiepileptic Drugs
Potential interactions between immediate-release topiramate and standard AEDs were assessed in controlled clinical pharmacokinetic studies in patients with epilepsy. The effects of these interactions on mean plasma AUCs are summarized in Table 12. Interaction of topiramate extended-release and standard AEDs is not expected to differ from the experience with immediate-release topiramate products.
In Table 12, the second column (AED concentration) describes what happens to the concentration of the co-administered AED listed in the first column when topiramate was added. The third column (topiramate concentration) describes how the co-administration of a drug listed in the first column modifies the concentration of topiramate when compared to topiramate given alone.
Table 12: Summary of AED Interactions with Topiramate|
AED |
AED |
Topiramate |
|---|---|---|
|
NC=Less than 10% change in plasma concentration | ||
| ||
|
Phenytoin |
NC or 25% increase * |
48% decrease |
|
Carbamazepine (CBZ) |
NC |
40% decrease |
|
CBZ epoxide † |
NC |
NE |
|
Valproic acid |
11% decrease |
14% decrease |
|
Phenobarbital |
NC |
NE |
|
Primidone |
NC |
NE |
|
Lamotrigine |
NC at TPM doses up to 400 mg per day |
13% decrease |
Oral Contraceptives
In a pharmacokinetic interaction study in healthy volunteers with a concomitantly administered combination oral contraceptive product containing 1 mg norethindrone (NET) plus 35 mcg ethinyl estradiol (EE), topiramate, given in the absence of other medications at doses of 50 to 200 mg per day, was not associated with statistically significant changes in mean exposure (AUC) to either component of the oral contraceptive. In another study, exposure to EE was statistically significantly decreased at doses of 200, 400, and 800 mg per day (18%, 21%, and 30%, respectively) when given as adjunctive therapy in patients taking valproic acid. In both studies, topiramate (50 mg per day to 800 mg per day) did not significantly affect exposure to NET and there was no significant dose-dependent change in EE exposure for doses of 50 to 200 mg per day. The clinical significance of the changes observed is not known [see Drug Interactions (7.4)] .
Digoxin
In a single-dose study, serum digoxin AUC was decreased by 12% with concomitant topiramate administration. The clinical relevance of this observation has not been established.
Hydrochlorothiazide
A drug interaction study conducted in healthy volunteers evaluated the steady- state pharmacokinetics of hydrochlorothiazide (HCTZ) (25 mg every 24 hours) and topiramate (96 mg every 12 hours) when administered alone and concomitantly. The results of this study indicate that topiramate C maxincreased by 27% and AUC increased by 29% when HCTZ was added to topiramate. The clinical significance of this change is unknown. The steady- state pharmacokinetics of HCTZ were not significantly influenced by the concomitant administration of topiramate. Clinical laboratory results indicated decreases in serum potassium after topiramate or HCTZ administration, which were greater when HCTZ and topiramate were administered in combination.
Metformin
A drug interaction study conducted in healthy volunteers evaluated the steady- state pharmacokinetics of metformin (500 mg every 12 hours) and topiramate in plasma when metformin was given alone and when metformin and topiramate (100 mg every 12 hours) were given simultaneously. The results of this study indicated that the mean metformin C maxand AUC 0–12hincreased by 18% and 25%, respectively, when topiramate was added. Topiramate did not affect metformin T max. The clinical significance of the effect of topiramate on metformin pharmacokinetics is not known. Oral plasma clearance of topiramate appears to be reduced when administered with metformin. The clinical significance of the effect of metformin on topiramate pharmacokinetics is unclear.
Pioglitazone
A drug interaction study conducted in healthy volunteers evaluated the steady- state pharmacokinetics of topiramate and pioglitazone when administered alone and concomitantly. A 15% decrease in the AUC τ,ssof pioglitazone with no alteration in C max,sswas observed. This finding was not statistically significant. In addition, a 13% and 16% decrease in C max,ssand AUC τ,ssrespectively, of the active hydroxy-metabolite was noted as well as a 60% decrease in C max,ssand AUC τ,ssof the active keto-metabolite. The clinical significance of these findings is not known.
Glyburide
A drug-drug interaction study conducted in patients with type 2 diabetes evaluated the steady-state pharmacokinetics of glyburide (5 mg per day) alone and concomitantly with topiramate (150 mg per day). There was a 22% decrease in C maxand a 25% reduction in AUC 24for glyburide during topiramate administration. Systemic exposure (AUC) of the active metabolites, 4- trans- hydroxy glyburide (M1) and 3- cis-hydroxyglyburide (M2), was also reduced by 13% and 15% and C maxwas reduced by 18% and 25%, respectively. The steady- state pharmacokinetics of topiramate were unaffected by concomitant administration of glyburide.
Lithium
In patients, the pharmacokinetics of lithium were unaffected during treatment with topiramate at doses of 200 mg per day; however, there was an observed increase in systemic exposure of lithium (27% for C maxand 26% for AUC) following topiramate doses up to 600 mg per day [see Drug Interactions (7.7)] .
Haloperidol
The pharmacokinetics of a single dose of haloperidol (5 mg) were not affected following multiple dosing of topiramate (100 mg every 12 hr) in 13 healthy adults (6 males, 7 females).
Amitriptyline
There was a 12% increase in AUC and C maxfor amitriptyline (25 mg per day) in 18 healthy subjects (9 males, 9 females) receiving 200 mg per day of topiramate.
Sumatriptan
Multiple dosing of topiramate (100 mg every 12 hours) in 24 healthy volunteers (14 males, 10 females) did not affect the pharmacokinetics of single-dose sumatriptan either orally (100 mg) or subcutaneously (6 mg).
Risperidone
When administered concomitantly with topiramate at escalating doses of 100, 250, and 400 mg per day, there was a reduction in risperidone systemic exposure (16% and 33% for steady-state AUC at the 250 and 400 mg per day doses of topiramate). No alterations of 9-hydroxyrisperidone levels were observed. Coadministration of topiramate 400 mg per day with risperidone resulted in a 14% increase in C maxand a 12% increase in AUC 12of topiramate. There were no clinically significant changes in the systemic exposure of risperidone plus 9-hydroxyrisperidone or of topiramate; therefore, this interaction is not likely to be of clinical significance.
Propranolol
Multiple dosing of topiramate (200 mg per day) in 34 healthy volunteers (17 males, 17 females) did not affect the pharmacokinetics of propranolol following daily 160 mg doses. Propranolol doses of 160 mg per day in 39 volunteers (27 males, 12 females) had no effect on the exposure to topiramate, at a dose of 200 mg per day of topiramate.
Dihydroergotamine
Multiple dosing of topiramate (200 mg per day) in 24 healthy volunteers (12 males, 12 females) did not affect the pharmacokinetics of a 1 mg subcutaneous dose of dihydroergotamine. Similarly, a 1 mg subcutaneous dose of dihydroergotamine did not affect the pharmacokinetics of a 200 mg per day dose of topiramate in the same study.
Diltiazem
Coadministration of diltiazem (240 mg Cardizem CD ®) with topiramate (150 mg per day) resulted in a 10% decrease in C maxand 25% decrease in diltiazem AUC, a 27% decrease in C maxand an 18% decrease in des-acetyl diltiazem AUC, and no effect on N-desmethyl diltiazem. Co-administration of topiramate with diltiazem resulted in a 16% increase in C maxand a 19% increase in AUC 12of topiramate.
Venlafaxine
Multiple dosing of topiramate (150 mg per day) in healthy volunteers did not affect the pharmacokinetics of venlafaxine or O-desmethyl venlafaxine. Multiple dosing of venlafaxine (150 mg) did not affect the pharmacokinetics of topiramate.
12.6 Relative Bioavailability of Topiramate Extended-Release Capsules
Compared to Immediate-Release Topiramate in Healthy Volunteers
Topiramate extended-release capsules, taken once daily, provides similar steady-state topiramate concentrations to immediate-release topiramate taken every 12 hours, when administered at the same total daily dose. In a healthy volunteer, multiple-dose crossover study, the 90% CI for the ratios of AUC 0–24, C maxand C min, as well as partial AUC (the area under the concentration-time curve from time 0 to time p (post dose)) for multiple time points were within the 80% to 125% bioequivalence limits, indicating no clinically significant difference between the two formulations. In addition, the 90% CI for the ratios of topiramate plasma concentration at each of multiple time points over 24 hours for the two formulations were within the 80% to 125% bioequivalence limits, except for the initial time points before 3 hours and at 8 hours post-dose, which is not expected to have a significant clinical impact.
The effects of switching between topiramate extended-release capsules and immediate-release topiramate were also evaluated in the same multiple-dose, crossover, comparative bioavailability study. In healthy subjects switched from immediate-release topiramate given every 12 hours to topiramate extended- release capsules given once daily, similar concentrations were maintained immediately after the formulation switch. On the first day following the switch, there were no significant differences in AUC 0–24, C max, and C min, as the 90% CI for the ratios were contained within the 80% to 125% equivalence limits.
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE | |||
|---|---|---|---|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 4/2025 | ||
|
What is the most important information I should know about topiramate
extended-release capsules?
These eye problems can lead to permanent loss of vision if not treated. You
should call your healthcare provider right away if you have any new eye
symptoms, including any new problems with your vision.
Your healthcare provider should do a blood test to measure the level of acid
in your blood before and during your treatment with topiramate extended-
release capsules. | |||
|
|
| |
|
Do not stop topiramate extended-release capsules without first talking to a healthcare provider.
How can I watch for early symptoms of suicidal thoughts and actions?
Topiramate extended-release capsules can harm your unborn baby.
Topiramate extended-release capsules may decrease the density of bones when
used over a long period. | |||
|
What is topiramate extended-release capsules?
| |||
|
What should I tell my healthcare provider before taking topiramate extended-
release capsules?
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Topiramate extended-release capsules and other medicines may affect each other
causing side effects.
Ask your healthcare provider if you are not sure if your medicine is listed
above. | |||
|
How should I take topiramate extended-release capsules?
| |||
|
What should I avoid while taking topiramate extended-release capsules?
| |||
|
What are the possible side effects of topiramate extended-release
capsules? ***High blood ammonia levels.**High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting. This has happened when topiramate extended-release is taken with a medicine called valproic acid (DEPAKENE ®and DEPAKOTE ®). ***Kidney stones.**Drink plenty of fluids when taking topiramate extended-release capsules to decrease your chances of getting kidney stones. ***Low body temperature.**Taking topiramate extended-release capsules when you are also taking valproic acid can cause a drop in body temperature to less than 95°F, or can cause tiredness, confusion, or coma. ***Effects on thinking and alertness.**Topiramate extended-release capsules may affect how you think, and cause confusion, problems with concentration, attention, memory, or speech. Topiramate extended-release capsules may cause depression or mood problems, tiredness, and sleepiness. *Dizziness or loss of muscle coordination. ***Serious skin reactions.**Topiramate extended-release capsules may cause a severe rash with blisters and peeling skin, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome). Topiramate extended-release capsules may also cause a rash with blisters and peeling skin over much of the body that may cause death (toxic epidermal necrolysis). Call your healthcare provider right away if you develop a skin rash or blisters. Call your healthcare provider right away if you have any of the symptoms
above. | |||
|
|
| |
|
Tell your healthcare provider about any side effect that bothers you or that
does not go away. | |||
|
You may also report side effects to Upsher-Smith Laboratories, LLC at 1-855-899-9180. | |||
|
How should I store topiramate extended-release capsules?
| |||
|
General information about the safe and effective use of topiramate extended-
release capsules. | |||
|
What are the ingredients in topiramate extended-release capsules? |
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Extended-Release: Bridging Study to Demonstrate Pharmacokinetic
Equivalence between Extended-Release and Immediate-Release Topiramate Formulations
Although a controlled clinical trial was performed (Study 14) [see Clinical Studies (14.4)] , the basis for approval of the extended-release formulation included the studies described below using an immediate-release formulation [see Clinical Studies (14.2, 14.3, 14.5)] and the demonstration of the pharmacokinetic equivalence of topiramate extended-release to immediate- release topiramate through the analysis of concentrations and cumulative AUCs at multiple time points [see Clinical Pharmacology (12.6)] .
14.2 Monotherapy Epilepsy
Patients with Partial-Onset or Primary Generalized Tonic-Clonic Seizures
Adults and Pediatric Patients 10 Years of Age and Older
The effectiveness of topiramate as initial monotherapy in adults and pediatric patients 10 years of age and older with partial-onset or primary generalized tonic-clonic seizures was established in a multicenter, randomized, double- blind, dose-controlled, parallel-group trial (Study 1).
Study 1 was conducted in 487 patients diagnosed with epilepsy (6 to 83 years of age) who had 1 or 2 well-documented seizures during the 3-month retrospective baseline phase who then entered the study and received topiramate 25 mg/day for 7 days in an open-label fashion. Forty-nine percent of subjects had no prior AED treatment and 17% had a diagnosis of epilepsy for greater than 24 months. Any AED therapy used for temporary or emergency purposes was discontinued prior to randomization. In the double-blind phase, 470 patients were randomized to titrate up to 50 mg/day or 400 mg/day of topiramate. If the target dose could not be achieved, patients were maintained on the maximum tolerated dose. Fifty-eight percent of patients achieved the maximal dose of 400 mg/day for >2 weeks, and patients who did not tolerate 150 mg/day were discontinued.
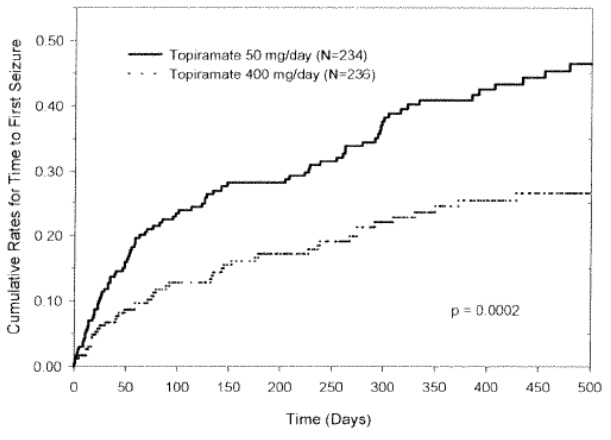
The primary efficacy assessment was a between-group comparison of time to first seizure during the double-blind phase. Comparison of the Kaplan-Meier survival curves of time to first seizure favored the topiramate 400 mg/day group over the topiramate 50 mg/day group (Figure 1). The treatment effects with respect to time to first seizure were consistent across various patient subgroups defined by age, sex, geographic region, baseline body weight, baseline seizure type, time since diagnosis, and baseline AED use.
Figure 1: Kaplan-Meier Estimates of Cumulative Rates for Time to First Seizure in Study 1
Pediatric Patients 2 to 9 Years of Age
The conclusion that topiramate is effective as initial monotherapy in pediatric patients 2 to 9 years of age with partial-onset or primary generalized tonic-clonic seizures was based on a pharmacometric bridging approach using data from the controlled epilepsy trials conducted with immediate-release topiramate described in labeling. This approach consisted of first showing a similar exposure-response relationship between pediatric patients down to 2 years of age and adults when immediate-release topiramate was given as adjunctive therapy. Similarity of exposure-response was also demonstrated in pediatric patients 6 to less than 16 years of age and adults when topiramate was given as initial monotherapy. Specific dosing in pediatric patients 2 to 9 years of age was derived from simulations utilizing plasma exposure ranges observed in pediatric and adult patients treated with immediate-release topiramate initial monotherapy [see Dosage and Administration (2.1)] .
14.3 Adjunctive Therapy Epilepsy
Adult Patients with Partial-Onset Seizures
The effectiveness of topiramate as an adjunctive treatment for adults with partial-onset seizures was established in six multicenter, randomized, double- blind, placebo-controlled trials (Studies 2, 3, 4, 5, 6, and 7), two comparing several dosages of topiramate and placebo and four comparing a single dosage with placebo, in patients with a history of partial-onset seizures, with or without secondarily generalized seizures.
Patients in these studies were permitted a maximum of two antiepileptic drugs (AEDs) in addition to topiramate tablets or placebo. In each study, patients were stabilized on optimum dosages of their concomitant AEDs during baseline phase lasting between 4 and 12 weeks. Patients who experienced a pre-specified minimum number of partial onset seizures, with or without secondary generalization, during the baseline phase (12 seizures for 12-week baseline, 8 for 8-week baseline or 3 for 4-week baseline) were randomly assigned to placebo or a specified dose of topiramate tablets in addition to their other AEDs.
Following randomization, patients began the double-blind phase of treatment. In five of the six studies, patients received active drug beginning at 100 mg per day; the dose was then increased by 100 mg or 200 mg/day increments weekly or every other week until the assigned dose was reached, unless intolerance prevented increases. In Study 7, the 25 or 50 mg/day initial doses of topiramate were followed by respective weekly increments of 25 or 50 mg/day until the target dose of 200 mg/day was reached. After titration, patients entered a 4, 8 or 12-week stabilization period. The numbers of patients randomized to each dose and the actual mean and median doses in the stabilization period are shown in Table 13.
Pediatric Patients 2 to 16 Years of Age with Partial-Onset Seizures
The effectiveness of topiramate as an adjunctive treatment for pediatric patients 2 to 16 years of age with partial-onset seizures was established in a multicenter, randomized, double-blind, placebo-controlled trial (Study 8), comparing topiramate and placebo in patients with a history of partial-onset seizures, with or without secondarily generalized seizures (see Table 14).
Patients in this study were permitted a maximum of two antiepileptic drugs (AEDs) in addition to topiramate tablets or placebo. In Study 8, patients were stabilized on optimum dosages of their concomitant AEDs during an 8-week baseline phase. Patients who experienced at least six partial-onset seizures, with or without secondarily generalized seizures, during the baseline phase were randomly assigned to placebo or topiramate tablets in addition to their other AEDs.
Following randomization, patients began the double-blind phase of treatment. Patients received active drug beginning at 25 or 50 mg/day; the dose was then increased by 25 mg to 150 mg/day increments every other week until the assigned dosage of 125, 175, 225, or 400 mg/day based on patients' weight to approximate a dosage of 6 mg/kg/day was reached, unless intolerance prevented increases. After titration, patients entered an 8-week stabilization period.
Patients with Primary Generalized Tonic-Clonic Seizures
The effectiveness of topiramate as an adjunctive treatment for primary generalized tonic-clonic seizures in patients 2 years of age and older was established in a multicenter, randomized, double-blind, placebo-controlled trial (Study 9), comparing a single dosage of topiramate and placebo (see Table 14).
Patients in Study 9 were permitted a maximum of two antiepileptic drugs (AEDs) in addition to topiramate or placebo. Patients were stabilized on optimum dosages of their concomitant AEDs during an 8-week baseline phase. Patients who experienced at least three primary generalized tonic-clonic seizures during the baseline phase were randomly assigned to placebo or topiramate in addition to their other AEDs.
Following randomization, patients began the double-blind phase of treatment. Patients received active drug beginning at 50 mg/day for four weeks; the dose was then increased by 50 mg to 150 mg/day increments every other week until the assigned dose of 175, 225, or 400 mg/day based on patients' body weight to approximate a dosage of 6 mg/kg/day was reached, unless intolerance prevented increases. After titration, patients entered a 12-week stabilization period.
Patients with Lennox-Gastaut Syndrome
The effectiveness of topiramate as an adjunctive treatment for seizures associated with Lennox-Gastaut syndrome in patients 2 years of age and older was established in a multicenter, randomized, double-blind, placebo-controlled trial (Study 10) comparing a single dosage of topiramate with placebo (see Table 14).
Patients in Study 10 were permitted a maximum of two antiepileptic drugs (AEDs) in addition to topiramate or placebo. Patients who were experiencing at least 60 seizures per month before study entry were stabilized on optimum dosages of their concomitant AEDs during a 4-week baseline phase. Following baseline, patients were randomly assigned to placebo or topiramate in addition to their other AEDs. Active drug was titrated beginning at 1 mg/kg/day for a week; the dose was then increased to 3 mg/kg/day for one week, then to 6 mg/kg/day. After titration, patients entered an 8-week stabilization period. The primary measures of effectiveness were the percent reduction in drop attacks and a parental global rating of seizure severity.
Table 13: Immediate-Release Topiramate Dose Summary During the Stabilization Periods of Each of Six Double-Blind, Placebo-Controlled, Adjunctive Trials in Adults with Partial-Onset Seizures *|
Target Topiramate Dosage (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
|
Study |
Stabilization Dose |
Placebo † |
200 |
400 |
600 |
800 |
1,000 |
| |||||||
|
N |
42 |
42 |
40 |
41 |
-- |
-- | |
|
2 |
Mean Dose |
5.9 |
200 |
390 |
556 |
-- |
-- |
|
Median Dose |
6.0 |
200 |
400 |
600 |
-- |
-- | |
|
N |
44 |
-- |
-- |
40 |
45 |
40 | |
|
3 |
Mean Dose |
9.7 |
-- |
-- |
544 |
739 |
796 |
|
Median Dose |
10.0 |
-- |
-- |
600 |
800 |
1,000 | |
|
N |
23 |
-- |
19 |
-- |
-- |
-- | |
|
4 |
Mean Dose |
3.8 |
-- |
395 |
-- |
-- |
-- |
|
Median Dose |
4.0 |
-- |
400 |
-- |
-- |
-- | |
|
N |
30 |
-- |
-- |
28 |
-- |
-- | |
|
5 |
Mean Dose |
5.7 |
-- |
-- |
522 |
-- |
-- |
|
Median Dose |
6.0 |
-- |
-- |
600 |
-- |
-- | |
|
N |
28 |
-- |
-- |
-- |
25 |
-- | |
|
6 |
Mean Dose |
7.9 |
-- |
-- |
-- |
568 |
-- |
|
Median Dose |
8 |
-- |
-- |
-- |
600 |
-- | |
|
N |
90 |
157 |
-- |
-- |
-- |
-- | |
|
7 |
Mean Dose |
8 |
200 |
-- |
-- |
-- |
-- |
|
Median Dose |
8 |
200 |
-- |
-- |
-- |
-- |
In all adjunctive topiramate trials, the reduction in seizure rate from baseline during the entire double-blind phase was measured. The median percent reductions in seizure rates and the responder rates (fraction of patients with at least a 50% reduction) by treatment group for each study are shown below in Table 14. As described above, a global improvement in seizure severity was also assessed in the Lennox-Gastaut trial.
Table 14: Efficacy Results in Double-Blind, Placebo-Controlled, Adjunctive Epilepsy Trials|
Target Topiramate Dosage (mg per day) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Study # |
Placebo |
200 |
400 |
600 |
800 |
1,000 |
≈6 mg/kg/day * | |
|
Comparisons with placebo: | ||||||||
Þ ß à è ð | ||||||||
|
Partial-Onset Seizures Studies in Adults | ||||||||
|
N |
45 |
45 |
45 |
46 |
-- |
-- |
-- | |
|
2 |
Median % Reduction |
12 |
27 † |
48 ‡ |
45 § |
-- |
-- |
-- |
|
% Responders |
18 |
24 |
44 ¶ |
46 ¶ |
-- |
-- |
-- | |
|
N |
47 |
-- |
-- |
48 |
48 |
47 |
-- | |
|
3 |
Median % Reduction |
2 |
-- |
-- |
41 § |
41 § |
36 § | |
|
% Responders |
9 |
-- |
-- |
40 § |
41 § |
36 ¶ | ||
|
N |
24 |
-- |
23 |
-- |
-- |
-- |
-- | |
|
4 |
Median % Reduction |
1 |
-- |
41 # |
-- |
-- |
-- |
-- |
|
% Responders |
8 |
-- |
35 ¶ |
-- |
-- |
-- |
-- | |
|
N |
30 |
-- |
-- |
30 |
-- |
-- |
-- | |
|
5 |
Median % Reduction |
-12 |
-- |
-- |
46 Þ |
-- |
-- |
-- |
|
% Responders |
10 |
-- |
-- |
47 § |
-- |
-- |
-- | |
|
N |
28 |
-- |
-- |
-- |
28 |
-- |
-- | |
|
6 |
Median % Reduction |
-21 |
-- |
-- |
-- |
24 § |
-- |
-- |
|
% Responders |
0 |
-- |
-- |
-- |
43 § |
-- |
-- | |
|
N |
91 |
168 |
-- |
-- |
-- |
-- |
-- | |
|
7 |
Median % Reduction |
20 |
44 § |
-- |
-- |
-- |
-- |
-- |
|
% Responders |
24 |
45 § | ||||||
|
Partial-Onset Seizures Studies in Pediatric Patients | ||||||||
|
N |
45 |
-- |
-- |
-- |
-- |
-- |
41 | |
|
8 |
Median % Reduction |
11 |
-- |
-- |
-- |
-- |
-- |
33 ¶ |
|
% Responders |
20 |
-- |
-- |
-- |
-- |
-- |
39 | |
|
Primary Generalized Tonic-Clonic****ß | ||||||||
|
N |
40 |
-- |
-- |
-- |
-- |
-- |
39 | |
|
9 |
Median % Reduction |
9 |
-- |
-- |
-- |
-- |
-- |
57 ¶ |
|
% Responders |
20 |
-- |
-- |
-- |
-- |
-- |
56 § | |
|
Lennox-Gastaut Syndrome****à | ||||||||
|
N |
49 |
-- |
-- |
-- |
-- |
-- |
46 | |
|
Median % Reduction |
-5 |
-- |
-- |
-- |
-- |
-- |
15 ¶ | |
|
10 |
% Responders |
14 |
28 è | |||||
|
Improvement in Seizure Severity ð |
28 |
52 ¶ |
Subset analyses of the antiepileptic efficacy of topiramate tablets in these studies showed no differences as a function of gender, race, age, baseline seizure rate, or concomitant AED.
In clinical trials for epilepsy, daily dosages were decreased in weekly intervals by 50 to 100 mg/day in adults and over a 2- to 8-week period in pediatric patients; transition was permitted to a new antiepileptic regimen when clinically indicated.
14.4 Extended-Release: Adjunctive Therapy in Adult Patients with Partial-
Onset Seizures with Topiramate
The effectiveness of topiramate extended-release as an adjunctive treatment for adults (18 to 75 years of age) was evaluated in a randomized, international, multi-center, double-blind, parallel-group, placebo-controlled trial in patients with a history of partial-onset seizures, with or without secondary generalization (Study 14).
Patients with partial-onset seizures on a stable dose of 1 to 3 AEDs entered into an 8-week baseline period. Patients who experienced at least 8 partial onset seizures, with or without secondary generalization, and no more than 21 consecutive seizure free days during the 8-week baseline phase were randomly assigned to placebo or topiramate extended-release administered once daily in addition to their concomitant AEDs. Following randomization, 249 patients began the double-blind treatment phase, which consisted of an initial 3-week titration period followed by an 8-week maintenance period. During the titration period, patients received topiramate extended-release or placebo beginning at 50 mg once daily; the dose was increased at weekly intervals by 50 mg once daily, or the placebo equivalent, until a final dose of 200 mg once daily was achieved. Patients then entered the maintenance period at the assigned dose of 200 mg once daily, or its placebo equivalent.
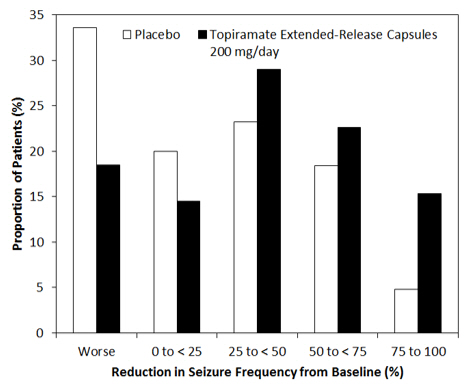
The percent reduction in the frequency of partial-onset seizure, baseline period compared to the treatment phase, was the primary endpoint. Data was analyzed by the Wilcoxon rank-sum test, with the criteria of statistical significance of p<0.05. The results of the analysis are presented in Table 15. The median percent reduction in seizure rate was 39.5% in patients taking topiramate extended-release (N=124) and 21.7% in patients taking placebo (N=125). This difference was statistically significant.
Table 15: Percent Reduction From Baseline in Partial-Onset Seizure Frequency During 11-week Treatment Period in Study 14|
Study End Point |
Topiramate extended-release |
Placebo |
|---|---|---|
| ||
|
Median Percent Reduction from Baseline * |
39.5% |
21.7% |
Figure 2 shows the change from baseline during titration plus maintenance (11 weeks) in partial-onset seizure frequency by category for patients treated with topiramate extended-release and placebo. Patients in whom the seizure frequency increased are shown as "worse." Patients in whom the seizure frequency decreased are shown in four categories of reduction in seizure frequency.
Figure 2: Proportion of Patients by Category of Seizure Response to Topiramate Extended-Release Capsules and Placebo
14.5 Preventive Treatment of Migraine
Adult Patients
The results of 2 multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trials conducted in the US (Study 11) or the US and Canada (Study 12) established the effectiveness of immediate-release topiramate in the preventive treatment of migraine. The design of both trials was identical, enrolling patients with a history of migraine, with or without aura, for at least 6 months, according to the International Headache Society (IHS) diagnostic criteria. Patients with a history of cluster headaches or basilar, ophthalmoplegic, hemiplegic, or transformed migraine headaches were excluded from the trials. Patients were required to have completed up to a 2-week washout of any prior migraine preventive medications before starting the baseline phase.
Patients who experienced 3 to 12 migraine headaches over the 4 weeks in the baseline phase were randomized to either topiramate 50 mg/day, 100 mg/day, 200 mg/day (twice the recommended daily dosage for the preventive treatment of migraine), or placebo and treated for a total of 26 weeks (8-week titration period and 18-week maintenance period). Treatment was initiated at 25 mg/day for one week, and then the daily dosage was increased by 25 mg increments each week until reaching the assigned target dose or maximum tolerated dose (administered twice daily).
Effectiveness of treatment was assessed by the reduction in migraine headache frequency, as measured by the change in 4-week migraine rate (according to migraines classified by IHS criteria) from the baseline phase to double-blind treatment period in each immediate-release topiramate treatment group compared to placebo in the Intent-To-Treat (ITT) population.
In Study 11, a total of 469 patients (416 females, 53 males), ranging in age from 13 to 70 years, were randomized and provided efficacy data. Two hundred sixty-five patients completed the entire 26-week double-blind phase. The median average daily dosages were 48 mg/day, 88 mg/day, and 132 mg/day in the target dose groups of topiramate 50, 100, and 200 mg/day, respectively.
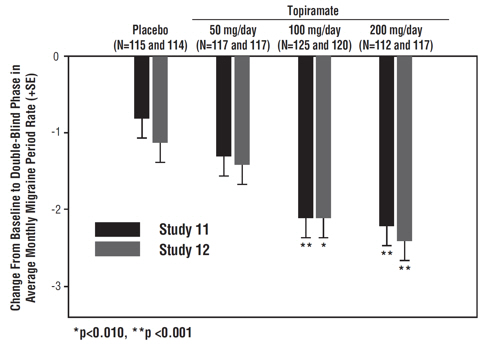
The mean migraine headache frequency rate at baseline was approximately 5.5 migraine headaches per 28 days and was similar across treatment groups. The change in the mean 4-week migraine headache frequency from baseline to the double-blind phase was -1.3, -2.1, and -2.2 in the immediate-release topiramate 50, 100, and 200 mg/day groups, respectively, versus -0.8 in the placebo group (see Figure 3). The treatment differences between the immediate- release topiramate 100 and 200 mg/day groups versus placebo were similar and statistically significant (p<0.001 for both comparisons).
In Study 12, a total of 468 patients (406 females, 62 males), ranging in age from 12 to 65 years, were randomized and provided efficacy data. Two hundred fifty-five patients completed the entire 26-week double-blind phase. The median average daily dosages were 47 mg/day, 86 mg/day, and 150 mg/day in the target dose groups of immediate-release topiramate 50, 100, and 200 mg/day, respectively.
The mean migraine headache frequency rate at baseline was approximately 5.5 migraine headaches per 28 days and was similar across treatment groups. The change in the mean 4-week migraine headache period frequency from baseline to the double-blind phase was -1.4, -2.1, and -2.4 in the immediate-release topiramate 50, 100, and 200 mg/day groups, respectively, versus -1.1 in the placebo group (see Figure 3). The differences between the immediate-release topiramate 100 and 200 mg/day groups versus placebo were similar and statistically significant (p=0.008 and p <0.001, respectively).
In both studies, there were no apparent differences in treatment effect within age or gender subgroups. Because most patients were Caucasian, there were insufficient numbers of patients from different races to make a meaningful comparison of race.
For patients withdrawing from immediate-release topiramate, daily dosages were decreased in weekly intervals by 25 to 50 mg/day.
Figure 3: Reduction in 4-Week Migraine Headache Frequency (Studies 11 and 12 for Adults and Adolescents)
Pediatric Patients 12 to 17 Years of Age
The effectiveness of immediate-release topiramate for the preventive treatment of migraine in pediatric patients 12 to 17 years of age was established in a multicenter, randomized, double-blind, parallel-group trial (Study 13). The study enrolled 103 patients (40 male, 63 female) 12 to 17 years of age with episodic migraine headaches with or without aura. Patient selection was based on IHS criteria for migraines (using proposed revisions to the 1988 IHS pediatric migraine criteria [IHS-R criteria]).
Patients who experienced 3 to 12 migraine attacks (according to migraines classified by patient reported diaries) and ≤14 headache days (migraine and non-migraine) during the 4-week prospective baseline period were randomized to either immediate-release topiramate 50 mg/day, 100 mg/day, or placebo and treated for a total of 16 weeks (4-week titration period followed by a 12-week maintenance period). Treatment was initiated at 25 mg/day for one week, and then the daily dosage was increased by 25 mg increments each week until reaching the assigned target dose or maximum tolerated dose (administered twice daily). Approximately 80% or more patients in each treatment group completed the study. The median average daily dosages were 45 and 79 mg/day in the target dose groups of immediate-release topiramate 50 and 100 mg/day, respectively.
Effectiveness of treatment was assessed by comparing each immediate-release topiramate treatment group to placebo (ITT population) for the percent reduction from baseline to the last 12 weeks of the double-blind phase in the monthly migraine attack rate (primary endpoint). The percent reduction from baseline to the last 12 weeks of the double-blind phase in average monthly migraine attack rate is shown in Table 16. The 100 mg immediate-release topiramate dose produced a statistically significant treatment difference relative to placebo of 28% reduction from baseline in the monthly migraine attack rate.
The mean reduction from baseline to the last 12 weeks of the double-blind phase in average monthly attack rate, a key secondary efficacy endpoint in Study 13 (and the primary efficacy endpoint in Studies 11 and 12, of adults) was 3.0 for 100 mg immediate-release topiramate dose and 1.7 for placebo. This 1.3 treatment difference in mean reduction from baseline of monthly migraine rate was statistically significant (p = 0.0087).
Table 16: Percent Reduction from Baseline to the Last 12 Weeks of Double-Blind Phase in Average Monthly Attack Rate: Study 13 (Intent-to-Treat Analysis Set)|
Category |
Placebo |
Topiramate 50 mg/day |
Topiramate 100 mg/day |
|---|---|---|---|
| |||
|
Baseline | |||
|
Median |
3.6 |
4.0 |
4.0 |
|
Last 12 Weeks of Double-Blind Phase | |||
|
Median |
2.3 |
2.3 |
1.0 |
|
Percent Reduction (%) | |||
|
Median |
44.4 |
44.6 |
72.2 |
|
P-value versus Placebo *,† |
0.7975 |
0.0164 ‡ |
