Ganciclovir
These highlights do not include all the information needed to use GANCICLOVIR FOR INJECTION safely and effectively. See full prescribing information for GANCICLOVIR FOR INJECTION.GANCICLOVIR FOR INJECTION, for intravenous useInitial U.S. Approval: 1989Rx only
35addab5-09db-4f8a-9c69-6fbce33ecc37
HUMAN PRESCRIPTION DRUG LABEL
Dec 21, 2022
Fresenius Kabi USA, LLC
DUNS: 608775388
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GANCICLOVIR SODIUM
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
BOXED WARNING SECTION
**WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY,
MUTAGENESIS AND CARCINOGENESIS**
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Treatment of CMV Retinitis
In a retrospective, non-randomized, single-center analysis of 41 patients with AIDS and CMV retinitis diagnosed by ophthalmologic examination between August 1983 and April 1988, treatment with Ganciclovir solution resulted in a delay in mean (median) time to first retinitis progression compared to untreated controls [105 (71) days from diagnosis vs 35 (29) days from diagnosis]. Patients in this series received induction treatment of Ganciclovir 5 mg/kg twice daily for 14 to 21 days followed by maintenance treatment with either 5 mg/kg once daily, 7 days per week or 6 mg/kg once daily, 5 days per week.
In a controlled, randomized study conducted between February 1989 and December 1990, immediate treatment with Ganciclovir was compared to delayed treatment in 42 patients with AIDS and peripheral CMV retinitis; 35 of 42 patients (13 in the immediate-treatment group and 22 in the delayed-treatment group) were included in the analysis of time to retinitis progression. Based on masked assessment of fundus photographs, the mean [95% CI] and median [95% CI] times to progression of retinitis were 66 days [39, 94] and 50 days [40, 84], respectively, in the immediate-treatment group compared to 19 days [11, 27] and 13.5 days [8, 18], respectively, in the delayed-treatment group.
Data from trials ICM 1653, ICM 1774, and AVI 034, which were performed comparing Ganciclovir for injection to oral ganciclovir for treatment of CMV retinitis in patients with AIDS, are shown in Table 12 and Figures 1, 2, and 3, and are discussed below.
Table 12. Population Characteristics in Studies ICM 1653, ICM 1774 and AVI 034|
Demographics |
ICM 1653 |
ICM 1774 |
AVI 034 | |
|
Median age (years) Range |
38 |
37 |
39 | |
|
Sex |
Males |
116 (96%) |
222 (99%) |
148 (93%) |
|
Females |
5 (4%) |
3 (1%) |
10 (6%) | |
|
Ethnicity |
Asian |
3 (3%) |
5 (2%) |
7 (4%) |
|
Black |
11 (9%) |
9 (4%) |
3 (2%) | |
|
Caucasian |
98 (81%) |
186 (83%) |
140 (88%) | |
|
Other |
9 (7%) |
25 (11%) |
8 (5%) | |
|
Median CD4 Count Range |
9.5 |
7.0 |
10.0 | |
|
Mean (SD) Observation Time (days) |
107.9 (43.0) |
97.6 (42.5) |
80.9 (47.0) |
Trial ICM 1653: In this randomized, open-label, parallel group trial, conducted between March 1991 and November 1992, patients with AIDS and newly diagnosed CMV retinitis received a 3-week induction course of Ganciclovir solution, 5 mg/kg twice daily for 14 days followed by 5 mg/kg once daily for 1 additional week. Following the 21-day intravenous induction course, patients with stable CMV retinitis were randomized to receive 20 weeks of maintenance treatment with either Ganciclovir solution, 5 mg/kg once daily, or ganciclovir capsules, 500 mg 6 times daily (3000 mg/day). The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 57 days [44, 70] and 29 days [28, 43], respectively, for patients on oral therapy compared to 62 days [50, 73] and 49 days [29, 61], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -5 days [-22, 12]. See Figure 1 for comparison of the proportion of patients remaining free of progression over time.
Trial ICM 1774: In this three-arm, randomized, open-label, parallel group trial, conducted between June 1991 and August 1993, patients with AIDS and stable CMV retinitis following from 4 weeks to 4 months of treatment with Ganciclovir solution were randomized to receive maintenance treatment with Ganciclovir solution, 5 mg/kg once daily, ganciclovir capsules, 500 mg 6 times daily, or ganciclovir capsules, 1000 mg three times daily for 20 weeks. The study showed that the mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 54 days [48, 60] and 42 days [31, 54], respectively, for patients on oral therapy compared to 66 days [56, 76] and 54 days [41, 69], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -12 days [-24, 0]. See Figure 2 for comparison of the proportion of patients remaining free of progression over time.
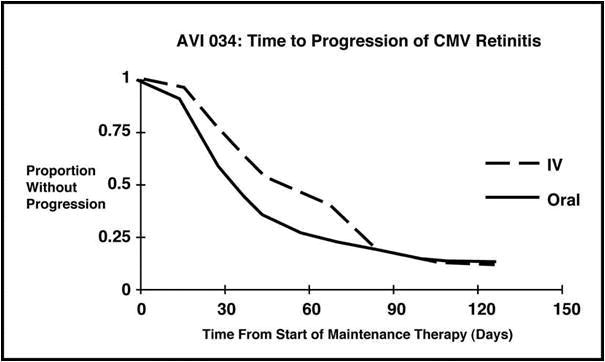
Trial AVI 034: In this randomized, open-label, parallel group trial, conducted between June 1991 and February 1993, patients with AIDS and newly diagnosed (81%) or previously treated (19%) CMV retinitis who had tolerated 10 to 21 days of induction treatment with Ganciclovir, 5 mg/kg twice daily, were randomized to receive 20 weeks of maintenance treatment with either ganciclovir capsules, 500 mg 6 times daily, or Ganciclovir solution, 5 mg/kg/day. The mean [95% CI] and median [95% CI] times to progression of CMV retinitis, as assessed by masked reading of fundus photographs, were 51 days [44, 57] and 41 days [31, 45], respectively, for patients on oral therapy compared to 62 days [52, 72] and 60 days [42, 83], respectively, for patients on intravenous therapy. The difference [95% CI] in the mean time to progression between the oral and intravenous therapies (oral - IV) was -11 days [-24, 1]. See Figure 3 for comparison of the proportion of patients remaining free of progression over time.
Comparison of other CMV retinitis outcomes between oral and IV formulations (development of bilateral retinitis, progression into Zone 1, and deterioration of visual acuity), while not definitive, showed no marked differences between treatment groups in these studies. Because of low event rates among these endpoints, these studies are underpowered to rule out significant differences in these endpoints.
Figure 1 Trial ICM 1653: Time to Progression of CMV Retinitis
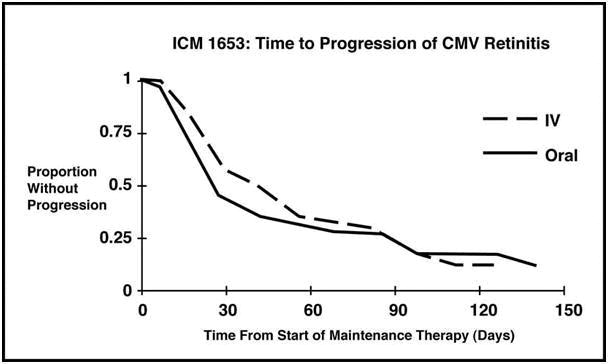
Figure 2 Trial ICM 1774: Time to Progression of CMV Retinitis
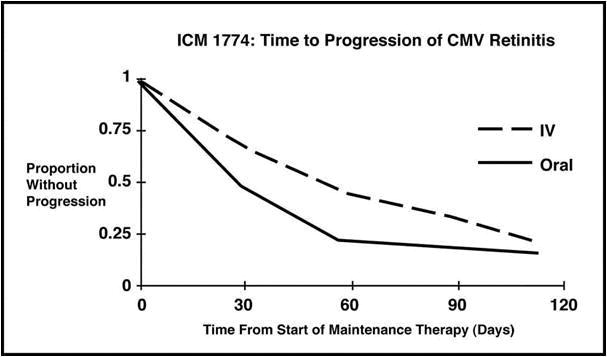
Figure 3 Trial AVI 034: Time to Progression of Retinitis
14.2 Prevention of CMV Disease in Transplant Recipients
Ganciclovir was evaluated in three randomized, controlled trials of prevention of CMV disease in organ transplant recipients.
Trial ICM 1496: In a randomized, double-blind, placebo-controlled study of 149 heart transplant recipients at risk for CMV infection (CMV seropositive or a seronegative recipient of an organ from a CMV seropositive donor), there was a reduction in the overall incidence of CMV disease in patients treated with Ganciclovir. Immediately post-transplant, patients received Ganciclovir solution 5 mg/kg twice daily for 14 days followed by 6 mg/kg once daily for 5 days/week for an additional 14 days. Twelve of the 76 (16%) patients treated with Ganciclovir vs 31 of the 73 (43%) placebo-treated patients developed CMV disease during the 120-day post-transplant observation period. No significant differences in hematologic toxicities were seen between the two treatment groups [see Adverse Reactions (6.1)].
Trial ICM 1689: In a randomized, double-blind, placebo-controlled study of 72 bone marrow transplant recipients with asymptomatic CMV infection (CMV positive culture of urine, throat or blood) there was a reduction in the incidence of CMV disease in patients treated with Ganciclovir following successful hematopoietic engraftment. Patients with virologic evidence of CMV infection received Ganciclovir solution 5 mg/kg twice daily for 7 days followed by 5 mg/kg once daily through day 100 post-transplant. One of the 37 (3%) patients treated with Ganciclovir vs 15 of the 35 (43%) placebo-treated patients developed CMV disease during the study. At 6 months post-transplant, there continued to be a reduction in the incidence of CMV disease in patients treated with Ganciclovir. Six of 37 (16%) patients treated with Ganciclovir vs 15 of the 35 (43%) placebo-treated patients developed disease through 6 months post-transplant. The overall rate of survival was higher in the group treated with Ganciclovir, both at day 100 and day 180 post-transplant. Although the differences in hematologic toxicities were not statistically significant, the incidence of neutropenia was higher in the group treated with Ganciclovir [see Adverse Reactions (6.1)].
Trial ICM 1570: This was a randomized, unblinded study that evaluated 40 allogeneic bone marrow transplant recipients at risk for CMV disease. Patients underwent bronchoscopy and bronchoalveolar lavage (BAL) on day 35 post- transplant. Patients with histologic, immunologic or virologic evidence of CMV infection in the lung were then randomized to observation or treatment with Ganciclovir solution (5 mg/kg twice daily for 14 days followed by 5 mg/kg once daily 5 days/week until day 120).
Four of 20 (20%) patients treated with Ganciclovir and 14 of 20 (70%) control patients developed interstitial pneumonia. The incidence of CMV disease was lower in the group treated with Ganciclovir, consistent with the results observed in ICM 1689.
REFERENCES SECTION
15 REFERENCES
- "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
