Prednisone
Revised: October 2015
13a6f44a-c0b9-48b0-8577-bc1120381a33
HUMAN PRESCRIPTION DRUG LABEL
Jun 7, 2023
RPK Pharmaceuticals, Inc.
DUNS: 147096275
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Prednisone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Prednisone tablets, USP contain prednisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. The chemical name for prednisone is pregna-1,4-diene-3,11,20-trione monohydrate, 17,21-dihydroxy-. The structural formula is represented below:
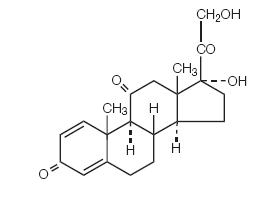
C21H26O5 M.W. 358.44
Prednisone is a white to practically white, odorless, crystalline powder. It is very slightly soluble in water; slightly soluble in alcohol, chloroform, dioxane, and methanol.
Each tablet, for oral administration, contains 5 mg, 10 mg or 20 mg of prednisone, USP (anhydrous). In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, crospovidone, docusate sodium, magnesium stearate and sodium benzoate.
Prednisone tablets, USP 20 mg also contain FD&C Yellow No. 6.
