Flucytosine
FLUCYTOSINE Capsules USPRx only
5721b46a-4a9f-46c2-8804-a9bad4d5f80a
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
Lupin Pharmaceuticals,Inc.
DUNS: 089153071
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Flucytosine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Flucytosine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
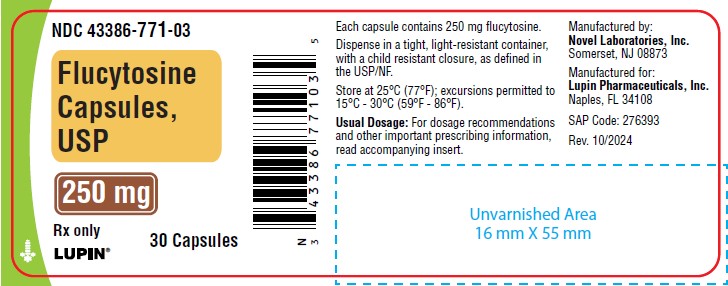
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Flucytosine Capsules USP, 250 mg
30 Capsules
Flucytosine Capsules USP, 500 mg
100 Capsules

DESCRIPTION SECTION
DESCRIPTION
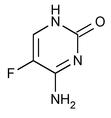
Flucytosine Capsules USP, an antifungal agent, is available as 250 mg and 500 mg capsules for oral administration. In addition to the active ingredient of flucytosine, each capsule contains lactose monohydrate, colloidal silicon dioxide, talc, sodium starch glycolate, magnesium stearate and hard gelatin capsule shell which contains gelatin, purified water, black iron oxide, FD&C Blue No.1, titanium dioxide and sodium lauryl sulfate for 250 mg strength; gelatin, purified water, black iron oxide and titanium dioxide for 500 mg strength. The imprinting ink contains black iron oxide, shellac, potassium hydroxide, titanium dioxide, povidone, sodium hydroxide and FD &C Red No. 40 aluminum lake for 250 mg and 500 mg strengths. Chemically, flucytosine is 5-fluorocytosine, a fluorinated pyrimidine which is related to fluorouracil and floxuridine. It is a white or almost white, crystalline powder with a molecular weight of 129.09 and the following structural formula:
HOW SUPPLIED SECTION
HOW SUPPLIED
Flucytosine Capsules USP, 250 mg are size 2 hard gelatin capsule with blue opaque cap and grey opaque body imprinted with "NL771" on the cap with black ink and "250" on the body with red ink filled with white to off white powder.
Bottles of 30 (NDC 43386-771-03)
Bottles of 100 (NDC 43386-771-01).
Flucytosine Capsules USP, 500 mg are size 0 hard gelatin capsule with grey opaque cap and white opaque body imprinted "NL770" on the cap with black ink and "500" on the body with red ink, filled with white to off white powder.
Bottles of 30 (NDC 43386-770-03)
Bottles of 100 (NDC 43386-770-01).
Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F).
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
SAP code: 276392
Rev: 10/2024
