LYUMJEV
These highlights do not include all the information needed to use LYUMJEV safely and effectively. See full prescribing information for LYUMJEV. LYUMJEV (insulin lispro-aabc) injection, for subcutaneous or intravenous useInitial U.S. Approval: 2020
c5a056e2-b568-4ca6-9ed8-79c010942d00
HUMAN PRESCRIPTION DRUG LABEL
Mar 29, 2023
Eli Lilly and Company
DUNS: 006421325
Products 6
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Insulin lispro-aabc
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a LYUMJEV Prefilled Pen, Cartridge, or Syringe Between
Patients
LYUMJEV prefilled pens or cartridges should never be shared between patients, even if the needle is changed. Patients using LYUMJEV vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin, insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6.1)].
Make any changes to a patient's insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, dosage adjustments of concomitant anti-diabetic products may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction associated with insulins, including LYUMJEV [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). LYUMJEV, or any insulin, should not be used during episodes of hypoglycemia [see Contraindications (4)].
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. As with all insulins, the glucose lowering effect time course of LYUMJEV may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Clinical Pharmacology (12.2)]. Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between LYUMJEV and other insulins, instruct patients to always check the insulin label before each injection.
Do not transfer LYUMJEV U-200 from the LYUMJEV KwikPen to a syringe. The markings on the insulin syringe will not measure the dose correctly and can result in overdosage and severe hypoglycemia [see Dosage and Administration (2.1) and Warnings and Precautions (5.3)].
5.5 Hypokalemia
All insulins, including LYUMJEV, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.6 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including LYUMJEV [see Adverse Reactions (6.1)]. If hypersensitivity reactions occur, discontinue LYUMJEV; treat per standard of care and monitor until symptoms and signs resolve. LYUMJEV is contraindicated in patients who have had hypersensitivity reactions to insulin lispro-aabc or any of its excipients [see Contraindications (4)].
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-Gamma
Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including LYUMJEV, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR- gamma agonist must be considered.
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of LYUMJEV may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16.2), and Patient Counseling Information (17)].
- Never share a LYUMJEV prefilled pen or cartridge between patients, even if the needle is changed. (5.1)
- Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient's insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of glucose monitoring. (5.2)
- Hypoglycemia: May be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness. (5.3)
- Hypoglycemia due to medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection. Do not transfer LYUMJEV U-200 from the LYUMJEV KwikPen to a syringe as overdosage and severe hypoglycemia can result. (5.4)
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated. (5.5)
- Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue LYUMJEV, monitor, and treat if indicated. (5.6)
- Fluid retention and heart failure with concomitant use of thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation of TZD if heart failure occurs. (5.7)
- Hyperglycemia and ketoacidosis due to insulin pump device malfunction: Monitor glucose and administer LYUMJEV by subcutaneous injection if pump malfunction occurs. (5.8)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 6 includes clinically significant drug interactions with LYUMJEV.
Table 6. Clinically Significant Drug Interactions with LYUMJEV|
Drugs That May Increase the Risk of Hypoglycemia | |
|
Drugs: |
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. |
|
Intervention: |
Dose reductions and increased frequency of glucose monitoring may be required when LYUMJEV is co-administered with these drugs. |
|
Drugs That May Decrease the Blood Glucose Lowering Effect of LYUMJEV | |
|
Drugs: |
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
|
Intervention: |
Dose increases and increased frequency of glucose monitoring may be required when LYUMJEV is co-administered with these drugs. |
|
Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of LYUMJEV | |
|
Drugs: |
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when LYUMJEV is co-administered with these drugs. |
|
Drugs That May Blunt Signs and Symptoms of Hypoglycemia | |
|
Drugs: |
Beta-blockers, clonidine, guanethidine, and reserpine. |
|
Intervention: |
Increased frequency of glucose monitoring may be required when LYUMJEV is co- administered with these drugs. |
- Drugs that Increase Hypoglycemia Risk or Increase or Decrease Blood Glucose Lowering Effect: Adjustment of dosage may be needed; closely monitor blood glucose. (7)
- Drugs that Blunt Hypoglycemia Signs and Symptoms (e.g., beta-blockers, clonidine, guanethidine, and reserpine): Increased frequency of glucose monitoring may be required. (7)
DESCRIPTION SECTION
11 DESCRIPTION
Insulin lispro-aabc is a rapid-acting human insulin analog used to lower blood glucose. Insulin lispro-aabc is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli. Insulin lispro-aabc differs from human insulin in that the amino acid proline at position B28 is replaced by lysine and the lysine in position B29 is replaced by proline. Chemically, it is Lys(B28), Pro(B29) human insulin analog and has the empirical formula C257H383N65O77S6 and a molecular weight of 5808 daltons, both identical to that of human insulin.
Insulin lispro-aabc has the following primary structure:
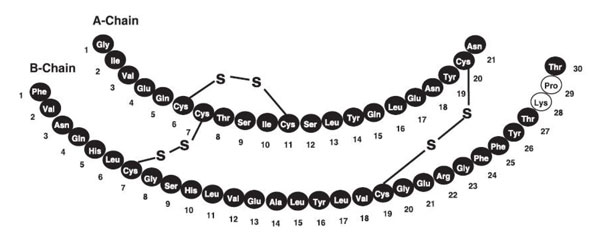
LYUMJEV (insulin lispro-aabc) injection is a sterile, aqueous, clear, and colorless solution for subcutaneous or intravenous administration. Each mL of LYUMJEV U-100 contains 100 units of insulin lispro-aabc and the inactive ingredients: glycerol (12.1 mg), magnesium chloride hexahydrate (1.02 mg), metacresol (3.15 mg), sodium citrate dihydrate (4.41 mg), treprostinil sodium (1.06 mcg), zinc oxide (content adjusted to provide 39 mcg zinc ion), and Water for Injection, USP.
Each mL of LYUMJEV U-200 contains 200 units of insulin lispro-aabc and the inactive ingredients: glycerol (12.1 mg), magnesium chloride hexahydrate (1.02 mg), metacresol (3.15 mg), sodium citrate dihydrate (4.41 mg), treprostinil sodium (1.06 mcg), zinc oxide (content adjusted to provide 52 mcg zinc ion), and Water for Injection, USP.
Hydrochloric acid and/or sodium hydroxide may be added to adjust the pH. LYUMJEV has a pH of 7.0 to 7.8.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of LYUMJEV is the regulation of glucose metabolism. Insulins, including insulin lispro-aabc, exert their specific action through binding to insulin receptors. Receptor-bound insulin lowers glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis, and enhance protein synthesis.
12.2 Pharmacodynamics
The time course of insulin action (i.e., glucose lowering) may vary considerably in different individuals or within the same individual. The average pharmacodynamic profile [i.e., glucose lowering effect measured as glucose infusion rate (GIR) in a euglycemic clamp study] for subcutaneous administration of 7, 15, and 30 units of LYUMJEV in 42 healthy subjects is shown in Figure 1 and key characteristics of the timing of the effect are described in Table 7 below.
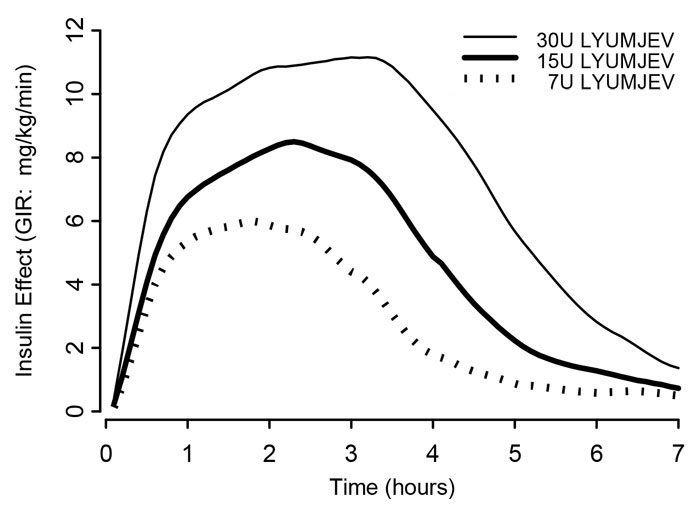
Figure 1. Mean Insulin Effect Over Time After Subcutaneous Administration of 7, 15, and 30 units of LYUMJEV in Healthy Subjects.
Table 7. Timing of Insulin Effect (i.e., Mean Pharmacodynamic Effect) After Subcutaneous Administration of 7, 15, and 30 Units of LYUMJEV in Healthy Subjects (N=42) and Corresponding to the Data Shown in Figure 1|
Parameter for Insulin Effect |
LYUMJEV |
LYUMJEV |
LYUMJEV |
|
Time to first measurable effect |
~17 minutes |
~17 minutes |
~15 minutes |
|
Time to peak effect |
~120 minutes |
~138 minutes |
~174 minutes |
|
Time for effect to return to baseline |
~4.6 hours |
~6.2 hours |
~7.3 hours |
On average, the pharmacodynamic effects of LYUMJEV, measured as area under the glucose infusion rate-time curve (AUCGIR), was 1080 mg/kg, 1860 mg/kg, and 3030 mg/kg following administration of 7, 15, and 30 units of LYUMJEV in healthy subjects.
Similar pharmacodynamic profiles were observed in separate studies conducted in 40 patients with type 1 diabetes and 38 patients with type 2 diabetes given LYUMJEV subcutaneously as a single 15 unit dose.
The onset and total glucose lowering were similar when LYUMJEV was administered in the abdomen, deltoid, or thigh. The day-to-day variability [percent coefficient of variation (CV%)] within subjects in the glucose- lowering-effect of LYUMJEV was 24% for the early glucose lowering (AUCGIR, 0-1h), 27% for the total glucose lowering (AUCGIR, 0-10h), and 19% for maximum glucose lowering effect (GIRmax).
Postprandial Glucose Lowering
When given at the start of a meal or 20 minutes after the start of the meal, LYUMJEV reduced postprandial glucose during a standardized test meal over the complete 5-hour period [change from premeal AUC(0-5h)] in patients with type 1 or type 2 diabetes.
The maximum and total glucose lowering were comparable for a single 15 unit dose of LYUMJEV 200 units/mL or LYUMJEV 100 units/mL when administered subcutaneously to healthy subjects. The insulin time action profile with LYUMJEV 200 units/mL was the same as observed with LYUMJEV 100 units/mL.
12.3 Pharmacokinetics
Absorption
Absorption of insulin lispro-aabc was evaluated in healthy subjects (see Figure 2) and patients with diabetes following subcutaneous injection of LYUMJEV.
- Insulin lispro-aabc appeared in circulation approximately 1 minute after injection of LYUMJEV.
- Time to 50% maximum insulin lispro-aabc concentration was 13 minutes.
- Time to maximum insulin lispro-aabc concentration was achieved at 57 minutes.
In healthy subjects, the day-to-day variability [CV%] within subjects of LYUMJEV was 10% for total exposure (AUC, 0-10h) and 16% for maximum insulin lispro-aabc concentration (Cmax).
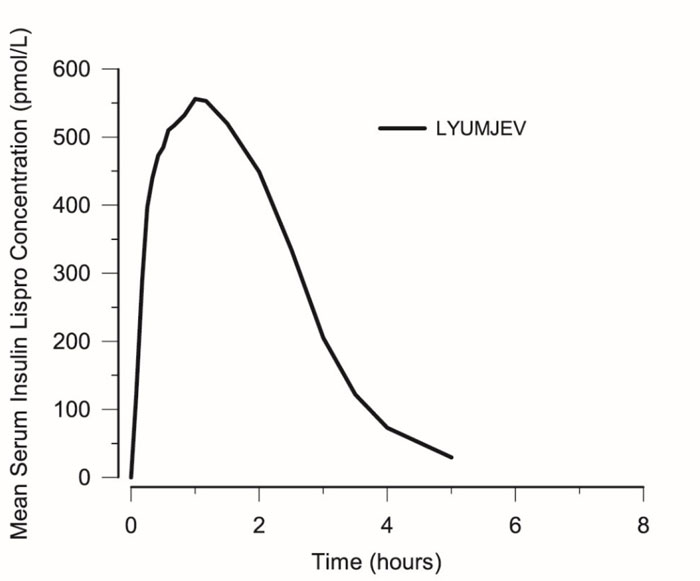
Figure 2. Mean Serum Insulin Lispro-aabc After Subcutaneous Injection of LYUMJEV (15 unit dose) in Healthy Subjects
The absolute bioavailability of insulin lispro-aabc after subcutaneous administration of LYUMJEV in the abdomen, deltoid, and thigh was approximately 65%. The rate of absorption of insulin lispro-aabc is maintained regardless of injection site. Maximum concentration and time to maximum concentration were comparable for the abdomen and upper arm regions; time to maximum concentration was longer and maximum concentration was lower for the thigh.
Total insulin lispro-aabc exposure and maximum insulin lispro-aabc concentration increased proportionally with increasing subcutaneous doses of LYUMJEV within the therapeutic dose range.
The results of a study in healthy subjects demonstrated that LYUMJEV 200 units/mL is bioequivalent to LYUMJEV 100 units/mL following administration of a single 15 unit dose for the area under the serum insulin lispro-aabc concentration-time curve from time zero to infinity and maximum insulin lispro-aabc concentration. The rate of insulin lispro-aabc absorption after administration of LYUMJEV 200 units/mL was similar as observed with LYUMJEV 100 units/mL.
Distribution
Following a 15 unit intravenous bolus injection of LYUMJEV in healthy subjects, the geometric mean (CV%) volume of distribution of insulin lispro- aabc (Vd) was 34 L (30%).
Elimination
Following a 15 unit intravenous bolus injection of LYUMJEV in healthy subjects, the geometric mean (CV%) clearance of insulin lispro-aabc was 32 L/hour (22%) and the median half-life of insulin lispro-aabc was 44 minutes.
Specific Populations
Age, biological sex, and race did not affect the pharmacokinetics and pharmacodynamics of LYUMJEV.
Patients with Renal and Hepatic Impairment
Renal and hepatic impairment is not known to impact the pharmacokinetics of insulin lispro-aabc. Insulin requirements may be reduced in the presence of renal or hepatic impairment.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of insulin lispro-aabc or of other insulin lispro products.
In a 26-week trial in adult patients with type 1 diabetes (Study PRONTO-T1D) [see Clinical Studies (14.2)], 49% of LYUMJEV-treated patients were anti-drug (insulin lispro-aabc) antibody (ADA)-positive at baseline, 91% of whom had cross-reactive antibodies with native insulin. During this 26-week period in this trial, 33% of LYUMJEV-treated patients had treatment-emergent ADA post- baseline (i.e., either new ADA or a 57% increase in assay signal over baseline), 75% of whom had cross-reactive antibodies with native insulin.
In a 26-week trial in adult patients with type 2 diabetes (Study PROTO-T2D) [see Clinical Studies (14.3)], 35% of LYUMJEV-treated patients were ADA- positive at baseline, 81% of whom had cross-reactive antibodies with native insulin. During this 26-week period in this trial, 31% of LYUMJEV-treated patients had treatment-emergent ADA post-baseline (i.e., either new ADA or a 57% increase in assay signal over baseline), 68% of whom had cross-reactive antibodies with native insulin.
In a 26-week trial in pediatric patients with type 1 diabetes (Study-PRONTO- PEDS) [see Clinical Studies (14.5)], 73% of LYUMJEV-treated patients were ADA- positive at baseline. Of these ADA-positive patients, 97% had cross-reactive antibodies with native insulin. During this 26-week period in this trial, 31% of LYUMJEV-treated patients had treatment-emergent ADA post-baseline (i.e., either new ADA or a 57% increase in assay signal over baseline). Of these treatment-emergent ADA-positive patients, 84% had cross-reactive antibodies with native insulin.
In these clinical trials, there were no identified clinically significant effects of ADA on safety or effectiveness (measured by HbA1c) of LYUMJEV over the treatment duration of 26-weeks.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In Fischer 344 rats, a 12-month repeat-dose toxicity study was conducted with insulin lispro at subcutaneous doses of 20 and 200 units/kg/day (approximately 3 and 32 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area). Insulin lispro did not produce important target organ toxicity including mammary tumors at any dose.
Insulin lispro was not mutagenic in the following genetic toxicity assays: bacterial mutation, unscheduled DNA synthesis, mouse lymphoma, chromosomal aberration, and micronucleus assays.
Male fertility was not compromised when male rats given subcutaneous insulin lispro injections of 5 and 20 units/kg/day (0.8 and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area) for 6 months were mated with untreated female rats. In a combined fertility, perinatal, and postnatal study in male and female rats given 1, 5, and 20 units/kg/day subcutaneously (0.2, 0.8, and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area), mating and fertility were not adversely affected in either gender at any dose.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The effectiveness of LYUMJEV was evaluated in:
- Two randomized, active controlled trials of 26 weeks in adults with type 1 diabetes (N=780) or type 2 diabetes (N=336) (PRONTO-T1D and PRONTO-T2D, respectively) [see Clinical Studies (14.2, 14.3)].
- A randomized, active controlled 16-week trial in adults with type 1 diabetes using continuous subcutaneous insulin infusion (N=432) (PRONTO-Pump-2) [see Clinical Studies (14.4)].
- A randomized, active controlled 26-week trial in pediatric patients with type 1 diabetes (N=716) (PRONTO-Peds) [see Clinical Studies (14.5)].
14.2 Adults with Type 1 Diabetes
PRONTO-T1D (NCT03214367) was a 26 week, randomized (4:4:3), active controlled, treat-to-target, multinational trial that evaluated the efficacy of LYUMJEV in 1222 adult patients with type 1 diabetes. Patients were randomized to either blinded mealtime LYUMJEV (N=451), blinded mealtime HUMALOG (N=442), or open- label postmeal LYUMJEV (N=329), all in combination with either insulin glargine or insulin degludec. Mealtime LYUMJEV or HUMALOG was injected 0 to 2 minutes before the meal and postmeal LYUMJEV was injected 20 minutes after the start of the meal.
Patients had a mean age of 44 years; mean duration of diabetes of 19 years; 56% were male; race: 77% White, 19% Asian, and 2% Black or African American. Eight percent of the randomized patients were Hispanic. The mean BMI was 26.6 kg/m2.
At week 26, treatment with mealtime LYUMJEV provided a mean reduction in HbA1c that met the pre-specified non-inferiority margin (0.4%) (see Table 8). In addition, postmeal LYUMJEV met the prespecified non-inferiority margin (0.4%) compared to mealtime HUMALOG. Insulin doses were similar in all treatment groups at baseline and at 26 weeks.
Table 8. Results from Study PRONTO-T1D: 26 Week Trial of Mealtime LYUMJEV and Postmeal LYUMJEV compared with Mealtime HUMALOG, all in Combination with Basal Insulin in Adults with Type 1 Diabetes|
a Analysis population: all randomized subjects regardless of adherence to treatment or availability of post-baseline assessment. Missing data at Week 26 were imputed by return to baseline approach. At week 26, primary efficacy assessment was missing for 3.8%, 4.8%, and 5.2% of subjects, for mealtime LYUMJEV, mealtime HUMALOG, and postmeal LYUMJEV, respectively. | |||
|
b Least-squares (LS) mean from ANCOVA adjusted for baseline value and other stratification factors. | |||
|
c Tested for non-inferiority. | |||
|
Mealtime LYUMJEV + basal insulin |
Mealtime HUMALOG + basal insulin |
Postmeal LYUMJEV + basal insulin | |
|
Number of randomized subjects (N) |
451 |
442 |
329 |
|
**HbA1c(%) (mean)**a | |||
|
Baseline |
7.3 |
7.3 |
7.4 |
|
Adjusted mean change from baselineb |
-0.12 |
-0.04 |
0.1 |
|
Estimated treatment difference versus |
-0.08 [-0.16, 0.00]c |
0.14 [0.05, 0.22] |
14.3 Adults with Type 2 Diabetes
PRONTO-T2D (NCT03214380) was a 26-week, randomized (1:1), active controlled, treat-to-target, multinational trial that evaluated the efficacy of LYUMJEV in 673 adult patients with type 2 diabetes who at study entry were on multiple daily injections with either basal insulin and at least one prandial insulin injection or premixed insulin with at least two injections daily. Patients may also have been treated with up to three oral anti-diabetic medications (OAMs) in addition to insulin. Patients were allowed to continue on metformin and/or a SGLT2 inhibitor and were randomized to either mealtime LYUMJEV (N=336) or to mealtime HUMALOG (N=337), both in combination with insulin glargine or insulin degludec in a basal-bolus regimen. Mealtime LYUMJEV or mealtime HUMALOG was injected 0-2 minutes before the meal.
Patients had a mean age of 61 years; mean duration of diabetes of 17 years; 53% were male; race: 69% White, 24% Asian, and 5% Black or African American. Twenty-three percent of the randomized patients were Hispanic. The mean BMI was 32.3 kg/m2.
At week 26, treatment with mealtime LYUMJEV provided a mean reduction of HbA1c from baseline that met the pre-specified non-inferiority margin (0.4%) compared to mealtime HUMALOG (see Table 9). Insulin doses were similar in both treatment groups at baseline and at 26 weeks.
Table 9. Results from Study PRONTO-T2D: 26 Week Trial of Mealtime LYUMJEV Compared with Mealtime HUMALOG, both in Combination with Basal Insulin in Adults with Type 2 Diabetes|
a Analysis population: all randomized subjects regardless of adherence to treatment or availability of post-baseline assessment. Missing data at Week 26 were imputed by return to baseline approach. At week 26, primary efficacy assessment was missing for 4.8% of subjects for mealtime LYUMJEV and for 4.5% mealtime HUMALOG. | ||
|
b Least-squares (LS) mean from ANCOVA adjusted for baseline value and other stratification factors. | ||
|
c Tested for non-inferiority. | ||
|
Mealtime LYUMJEV + basal insulin |
Mealtime HUMALOG + basal insulin | |
|
Number of randomized subjects (N) |
336 |
337 |
|
**HbA1c (%)**a | ||
|
Baseline mean |
7.3 |
7.3 |
|
Adjusted mean change from baselineb |
-0.36 |
-0.38 |
|
Estimated treatment difference versus |
0.03 [-0.08, 0.13] |
14.4 Adults with Type 1 Diabetes – Continuous Subcutaneous Insulin Infusion
(CSII)
PRONTO-Pump-2 (NCT03830281) was a 16 week randomized (1:1), active controlled, treat-to-target, multinational trial that evaluated the efficacy of LYUMJEV in 432 adult patients with type 1 diabetes currently using continuous subcutaneous insulin infusion. Patients were randomized to either blinded LYUMJEV (N=215) or blinded HUMALOG (N= 217). Mealtime LYUMJEV or HUMALOG boluses were initiated 0 to 2 minutes before the meal.
Patients had a mean age of 46 years; mean duration of diabetes of 26 years; 45% were male; race: 95% White, 3% Black or African American, and 0.5% Asian. Eight percent of the randomized patients were Hispanic. The mean BMI was 27.1 kg/m2.
At week 16, treatment with LYUMJEV provided a mean reduction in HbA1c that met the pre-specified non-inferiority margin (0.4%) compared to mealtime HUMALOG (see Table 10). Total daily insulin doses were similar for both treatment groups at baseline and at 16 weeks.
Table 10. Results from Study PRONTO-Pump-2: 16 Week Trial of LYUMJEV compared with HUMALOG in Adults with Type 1 Diabetes|
a Analysis population: all randomized subjects regardless of adherence to treatment or availability of post-baseline assessment. Missing data at Week 16 were imputed by return to baseline approach. At week 16, primary efficacy assessment was missing for 7% and 4.6% of subjects, for LYUMJEV and HUMALOG, respectively. | ||
|
b Least-squares (LS) mean from ANCOVA adjusted for baseline value and other stratification factors. | ||
|
c Tested for non-inferiority. | ||
|
LYUMJEV |
HUMALOG | |
|
Number of randomized subjects (N) |
215 |
217 |
|
**HbA1c (%)**a | ||
|
Baseline mean |
7.6 |
7.5 |
|
Adjusted mean change from baselineb |
-0.06 |
-0.09 |
|
Estimated treatment difference versus HUMALOG [95% CI]c |
0.03 [-0.05, 0.11] |
14.5 Pediatric Patients with Type 1 Diabetes
PRONTO-Peds (NCT03740919) was a 26-week, randomized (2:2:1), active controlled, treat-to-target, multinational trial that evaluated the efficacy of LYUMJEV in 716 pediatric patients with type 1 diabetes. Patients were randomized to either blinded mealtime LYUMJEV (N=280), blinded mealtime HUMALOG (N=298), or open-label postmeal LYUMJEV (N=138), all in combination with basal insulin (insulin glargine, insulin degludec or insulin detemir). Mealtime LYUMJEV or HUMALOG was injected 0 to 2 minutes before the meal and postmeal LYUMJEV was injected within 20 minutes after the start of the meal.
Patients had a mean age of 12 years (3-17 years); 51% were male; race: 89% White, 6% Asian, and 2% Black or African American. In the U.S. subpopulation in this trial, 24% of the randomized patients were Hispanic. The mean BMI was 20.4 kg/m2 and the mean duration of diabetes was 5 years.
At week 26, treatment with mealtime LYUMJEV provided a mean change in HbA1c that met the pre-specified non-inferiority margin (0.4%) (see Table 11). In addition, postmeal LYUMJEV met the prespecified non-inferiority margin (0.4%) compared to mealtime HUMALOG. Insulin doses were similar in all treatment groups at baseline and at 26 weeks.
Table 11. Results from Study PRONTO-Peds: 26 Week Trial of Mealtime LYUMJEV and Postmeal LYUMJEV compared with Mealtime HUMALOG, all in Combination with Basal Insulin in Pediatric Patients with Type 1 Diabetes|
a Analysis population: all randomized subjects regardless of adherence to treatment or availability of post-baseline assessment. Missing data at Week 26 were imputed by return to baseline approach. At week 26, primary efficacy assessment was missing for 5.4%, 7.1%, and 5.1% of subjects, for mealtime LYUMJEV, mealtime HUMALOG, and postmeal LYUMJEV, respectively. | |||
|
b Least-squares (LS) mean from ANCOVA adjusted for baseline value and other stratification factors. | |||
|
c Tested for non-inferiority. | |||
|
Mealtime LYUMJEV + basal insulin |
Mealtime HUMALOG + basal insulin |
Postmeal LYUMJEV + basal insulin | |
|
Number of randomized subjects (N) |
280 |
298 |
138 |
|
**HbA1c (%) (mean)**a | |||
|
Baseline |
7.8 |
7.8 |
7.8 |
|
Adjusted mean change from baselineb |
0.06 |
0.06 |
0.06 |
|
Estimated treatment difference versus |
-0.01 [-0.15, 0.14] |
-0.00 [-0.18, 0.18] |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
LYUMJEV (insulin lispro-aabc) injection is a clear and colorless solution available as shown in Table 12.
Table 12. How Supplied|
a 3 mL cartridge is for use in Eli Lilly and Company's HumaPen® Luxura® HD insulin delivery device. Patients need to check their device manual to determine if the LYUMJEV cartridge is compatible for use in other devices. | |||||
|
b Tempo Pen contains a component that allows for data connectivity when used with a compatible transmitter. | |||||
|
LYUMJEV |
NDC Number |
Concentration |
Total Units in Presentation |
Dose Increment |
Package Size |
|
U-100 multiple-dose 10 mL vial |
0002-7728-01 |
100 units/mL |
1,000 units |
n/a |
1 vial |
|
U-100 single-patient-use 3 mL cartridgea |
0002-7726-05 |
100 units/mL |
300 units |
n/a |
5 cartridges |
|
U-100 single-patient-use 3 mL KwikPen |
0002-8207-05 |
100 units/mL |
300 units |
1 unit |
5 pens |
|
U-100 single-patient-use 3 mL Junior KwikPen |
0002-8351-05 |
100 units/mL |
300 units |
0.5 unit |
5 pens |
|
U-100 single-patient-use 3 mL Tempo Penb |
0002-8235-05 |
100 units/mL |
300 units |
1 unit |
5 pens |
|
U-200 single-patient-use 3 mL KwikPen |
0002-8228-27 |
200 units/mL |
600 units |
1 unit |
2 pens |
16.2 Storage and Handling
- Dispense in the original sealed carton with the enclosed Instructions for Use.
- Refrigerate unopened LYUMJEV vials, pens, and cartridges between 36°F to 46°F (2°C to 8°C) until time of use and keep in the original carton to protect from light. Do not freeze or use LYUMJEV if it has been frozen. Do not expose to direct heat. Discard opened or unopened LYUMJEV vials, pens, and cartridges stored at room temperature below 86°F (30°C) after 28 days.
The storage conditions for vials, pens, and cartridges are summarized in Table 13.
Table 13. Storage Conditions for Vials, Pens, and Cartridges|
a In-use (opened) vials, whether or not refrigerated, must be used within 28 days. | ||||
|
b When stored at room temperature, LYUMJEV can only be used for a total of 28 days including both not in-use (unopened) and in-use (opened) storage time. | ||||
|
LYUMJEV Presentation |
Not In-use |
In-use | ||
|
Room Temperature (below 86°F [30°C]) |
Refrigerated |
Room Temperature (below 86°F [30°C]) |
Refrigerated | |
|
10 mL viala,b |
28 days |
Until expiration date |
28 days |
28 days |
|
3 mL cartridgeb |
28 days |
Until expiration date |
28 days |
Do not refrigerate |
|
3 mL LYUMJEV KwikPen (U-100 and U-200)b |
28 days |
Until expiration date |
28 days |
Do not refrigerate |
|
3 mL LYUMJEV Junior KwikPenb |
28 days |
Until expiration date |
28 days |
Do not refrigerate |
|
3 mL LYUMJEV Tempo Penb |
28 days |
Until expiration date |
28 days |
Do not refrigerate |
Storage of LYUMJEV in Insulin Pump
Change the LYUMJEV U-100 in the pump reservoir at least every 9 days, or according to the pump user manual, whichever is shorter, or after exposure to temperatures that exceed 98.6°F (37°C).
Storage of LYUMJEV in Intravenous Infusion Fluids
Diluted LYUMJEV may be stored for up to 4 days when refrigerated at 36°F to 46°F (2°C to 8°C) until time of use. The same solution may be stored up to 12 hours at room temperature at 68°F to 77°F (20°C to 25°C) [see Dosage and Administration (2.2)].
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Always check insulin labels before administration [see Warnings and Precautions (5.4)].
- Inspect LYUMJEV visually before use. It should appear clear and colorless. Do not use LYUMJEV if particulate matter and discoloration is seen.
- Use LYUMJEV prefilled pens with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
- Do not perform dose conversion when using any LYUMJEV U-100 or U-200 prefilled pens.The dose window of LYUMJEV prefilled pens shows the number of units of LYUMJEV to be delivered and no conversion is needed.
- Do not transfer LYUMJEV U-200 from the prefilled pen to a syringe for administration [see Warnings and Precautions (5.4)].
- Do not mix LYUMJEV with any other insulin products.
- Do not administer LYUMJEV U-200 using continuous subcutaneous infusion insulin pump.
- Do not administer LYUMJEV U-200 intravenously.
2.2 Route of Administration Instructions
Subcutaneous Injection for LYUMJEV U-100 or U-200
- Administer LYUMJEV at the start of a meal or within 20 minutes after starting a meal subcutaneously into the abdomen, upper arm, thigh, or buttocks.
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Adverse Reactions (6.1, 6.2)].
- LYUMJEV given by subcutaneous injection should generally be used in regimens with intermediate or long-acting insulin.
- The LYUMJEV U-100 KwikPen, LYUMJEV U-100 Tempo Pen, and LYUMJEV U-200 KwikPen each dial in 1 unit increments and deliver a maximum dose of 60 units per injection.
- The LYUMJEV U-100 Junior KwikPen dials in 0.5 unit increments and delivers a maximum dose of 30 units per injection.
Continuous Subcutaneous Insulin Infusion (Insulin Pump) for LYUMJEV U-100 Only
- Do not administer LYUMJEV U-200 using an insulin pump.
- Refer to the continuous subcutaneous insulin infusion pump user manual to see if LYUMJEV can be used with the insulin pump. Use LYUMJEV in accordance with the insulin pump system's instructions for use.
- Administer LYUMJEV U-100 by continuous subcutaneous infusion in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not infuse into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
- Train patients using continuous subcutaneous insulin infusion (CSII) therapy to administer insulin by injection and have alternate insulin therapy available in case of insulin pump failure [see Warnings and Precautions (5.8)].
- Change LYUMJEV U-100 in the pump reservoir at least every 9 days or according to the pump user manual, whichever is shorter.
- Change the infusion sets and the infusion set insertion site according to the manufacturer's user manual.
- Do not dilute or mix LYUMJEV U-100 when administering by CSII.
- Do not expose LYUMJEV in the pump reservoir to temperatures greater than 98.6°F (37°C).
Intravenous Administration for LYUMJEV U-100 Only
- Do not administer LYUMJEV U-200 intravenously.
- Administer LYUMJEV U-100 intravenously only under medical supervision with close monitoring of glucose and potassium levels to avoid hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)].
- Dilute LYUMJEV U-100 to a concentration of 1 unit/mL using 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP infusion solutions. Dilutions to concentrations below 1 unit/mL are not recommended.
- Diluted LYUMJEV may be stored for up to 4 days when refrigerated or up to 12 hours at room temperature [see HOW SUPPLIED/STORAGE AND HANDLING (16.2)].
2.3 General Dosage Instructions
- Individualize and adjust the dosage of LYUMJEV based on the patient's metabolic needs, glucose monitoring results, and glycemic control goal.
- If converting from another mealtime insulin to LYUMJEV, the change can be done on a unit-to-unit basis.
- Dosage adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycemia or hyperglycemia [see Warnings and Precautions (5.2, 5.3), Drug Interactions (7) and Use in Specific Populations (8.6, 8.7)].
- During changes to a patient's insulin regimen, increase the frequency of glucose monitoring [see Warnings and Precautions (5.2)].
- Instruct patients who forget a mealtime dose to monitor their glucose level to decide if an insulin dose is needed, and to resume their usual dosing schedule at the next meal.
- See Full Prescribing Information for important administration instructions. (2.1, 2.2)
- Subcutaneous Injection (2.2):
- Administer LYUMJEV U-100 or U-200 at the start of a meal or within 20 minutes after starting a meal subcutaneously into the abdomen, upper arm, thigh, or buttocks.
- Rotate injection sites within the same region to reduce risk of lipodystrophy and localized cutaneous amyloidosis.
- Should generally be used in regimens with an intermediate or long-acting insulin.
- Continuous subcutaneous infusion (Insulin Pump) (2.2):
- Refer to the insulin infusion pump user manual to see if LYUMJEV can be used. Use in accordance with the insulin pump instructions for use.
- Administer LYUMJEV U-100 by continuous subcutaneous infusion using an insulin pump in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis.
- Do not administer LYUMJEV U-200 by continuous subcutaneous infusion.
- Intravenous Infusion (2.2):
- Administer LYUMJEV U-100 intravenously only under medical supervision. DO NOT administer LYUMJEV U-200 by intravenous infusion.
- Dilute LYUMJEV U-100 to a concentration of 1 unit/mL.
- Individualize and adjust the dosage of LYUMJEV based on the patient's metabolic needs, glucose monitoring results, and glycemic control goal. (2.3)
- Dose adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns (i.e., amount and type of food, timing of food intake), changes in renal or hepatic function, or during acute illness. (2.3)
