BYLVAY
These highlights do not include all the information needed to use BYLVAY safely and effectively. See full prescribing information for BYLVAY. BYLVAY (odevixibat) capsules, for oral use BYLVAY (odevixibat) oral pellets Initial U.S. Approval: 2021
151f1d3e-2bf4-47ce-b1be-a892de3258fa
HUMAN PRESCRIPTION DRUG LABEL
Jun 15, 2023
Albireo Pharma, Inc.
DUNS: 145773359
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
odevixibat
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
odevixibat
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
odevixibat
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
odevixibat
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Progressive Familial Intrahepatic Cholestasis (PFIC)
BYLVAY is indicated for the treatment of pruritus in patients 3 months of age and older with PFIC.
Limitations of Use
- BYLVAY may not be effective in a subgroup of PFIC type 2 patients with specific ABCB11 variants resulting in non-functional or complete absence of the bile salt export pump protein [see Clinical Pharmacology (12.5) and Clinical Studies (14)].
1.2 Alagille Syndrome (ALGS)
BYLVAY is indicated for the treatment of cholestatic pruritus in patients 12 months of age and older with ALGS.
BYLVAY is an ileal bile acid transporter (IBAT) inhibitor indicated for:
Progressive Familial Intrahepatic Cholestasis (PFIC)
- the treatment of pruritus in patients 3 months of age and older with progressive familial intrahepatic cholestasis (PFIC). (1.1)
Limitation of Use:
BYLVAY may not be effective in a subgroup of PFIC type 2 patients with specific ABCB11 variants resulting in non-functional or complete absence of the bile salt export pump protein. (1.1, 12.5, 14)
Alagille Syndrome (ALGS)
- the treatment of cholestatic pruritus in patients 12 months of age and older with Alagille syndrome (ALGS). (1.2)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Liver Test Abnormalities
Patients enrolled in the PFIC trial had abnormal liver tests at baseline. In Trial 1 in PFIC patients, treatment-emergent elevations of liver tests or worsening of liver tests relative to baseline values were observed during the clinical trial. Most abnormalities included elevation in AST, ALT, or total and direct bilirubin (see Table 3). Treatment interruption days ranged from 3 days to 124 days; none of the patients in Trial 1 permanently discontinued treatment due to liver test abnormalities [see Adverse Reactions (6.1)].
Table 3. Treatment-Emergent Elevation in Liver Tests in Trial 1 (PFIC)|
Number of Patients with: |
Placebo |
BYLVAY 40 mcg/kg |
BYLVAY 120 mcg/kg |
|---|---|---|---|
|
ALT= alanine aminotransferase; AST= aspartate aminotransferase; DB= direct bilirubin; TB= total bilirubin; ULN= Upper Limit of Normal | |||
|
ALT increase over baseline ≥150 U/L |
0 |
2 (9%) |
2 (11%) |
|
AST increase over baseline by ≥150 U/L |
0 |
1 (4%) |
3 (16%) |
|
TB increase over baseline by ≥2 mg/dL |
1 (5%) |
4 (17%) |
1 (5%) |
|
DB increase over baseline by ≥1 mg/dL |
2 (10%) |
5 (22%) |
2 (11%) |
Patients enrolled in the ALGS trial had abnormal liver tests at baseline. In Trial 3 in ALGS patients, treatment-emergent elevations or worsening in liver tests relative to baseline values were observed during the trial. Most abnormalities included elevation in ALT or AST (see Table 4). One patient interrupted treatment for 40 days; none of the patients in Trial 3 permanently discontinued treatment due to liver test abnormalities.
Table 4. Treatment-Emergent Elevation in Liver Tests in Trial 3 (ALGS)|
Number of Patients with: |
Placebo |
BYLVAY 120 mcg/kg |
|---|---|---|
|
ALT= alanine aminotransferase; AST= aspartate aminotransferase; DB= direct bilirubin; TB= total bilirubin; ULN= Upper Limit of Normal | ||
|
ALT increase over baseline ≥150 U/L |
1 (6%) |
13 (37%) |
|
AST increase over baseline by ≥150 U/L |
2 (12%) |
9 (26%) |
|
TB increase over baseline by ≥2 mg/dL |
2 (12%) |
5 (14%) |
|
DB increase over baseline by ≥1 mg/dL |
4 (24%) |
6 (17%) |
Obtain baseline liver tests and monitor during treatment. Dose reduction or treatment interruption may be required if abnormalities occur. For persistent or recurrent liver test abnormalities, consider treatment discontinuation [see Dosage and Administration (2.3)].
BYLVAY was not evaluated in PFIC or ALGS patients with cirrhosis. Closely monitor for liver test abnormalities; permanently discontinue BYLVAY if a patient progresses to portal hypertension or experiences a hepatic decompensation event.
5.2 Diarrhea
In Trial 1, diarrhea in PFIC patients was reported in 2 (10%) placebo-treated patients, 9 (39%) BYLVAY-treated 40 mcg/kg/day patients and 4 (21%) BYLVAY- treated 120 mcg/kg/day patients. Treatment interruption due to diarrhea occurred in 2 patients with 3 events during treatment with BYLVAY 120 mcg/kg/day. Treatment interruption due to diarrhea ranged between 3 to 7 days [see Adverse Reactions (6.1)]. One patient treated with BYLVAY 120 mcg/kg/day withdrew from Trial 1 due to persistent diarrhea.
In Trial 3, diarrhea in ALGS patients was reported in 1 placebo-treated patient (6%) and in 10 (29%) BYLVAY-treated patients [see Adverse Reactions (6.1)]. No patients interrupted or permanently discontinued BYLVAY due to diarrhea.
If diarrhea occurs, monitor for dehydration and treat promptly. Interrupt BYLVAY dosing if a patient experiences persistent diarrhea. Restart BYLVAY at 40 mcg/kg/day when diarrhea resolves, and increase the dose as tolerated if appropriate. If diarrhea persists and no alternate etiology is identified, stop BYLVAY treatment.
5.3 Fat-Soluble Vitamin (FSV) Deficiency
Fat-soluble vitamins (FSV) include vitamin A, D, E, and K (measured using INR levels). PFIC and ALGS patients can have FSV deficiency at baseline, as part of their disease. BYLVAY may affect absorption of fat-soluble vitamins. In Trial 1, new onset or worsening of existing FSV deficiency was reported in 1 (5%) placebo-treated patient, and 3 (16%) BYLVAY-treated 120 mcg/kg/day patients; none of the BYLVAY-treated 40 mcg/kg/day patients had new onset or worsening of existing FSV deficiency. In Trial 3, new or worsening of existing FSV deficiency was reported in 3 (17.6%) placebo-treated patients and 3 (8.6%) BYLVAY-treated patients [see Adverse Reactions (6.1)].
Obtain serum FSV levels at baseline and monitor during treatment, along with any clinical manifestations. If FSV deficiency is diagnosed, supplement with FSV. Discontinue BYLVAY if FSV deficiency persists or worsens despite adequate FSV supplementation.
- Liver Test Abnormalities: Obtain baseline liver tests and monitor during treatment. Dose reduction or treatment interruption may be required if abnormalities occur. For persistent or recurrent liver test abnormalities, consider treatment discontinuation. (2.3, 5.1)
- Diarrhea: Treat dehydration. Treatment interruption or discontinuation may be required for persistent diarrhea. (5.2)
- Fat-Soluble Vitamin (FSV) Deficiency: Obtain baseline levels and monitor during treatment. Supplement if deficiency is observed. If FSV deficiency persists or worsens despite FSV supplementation, discontinue treatment. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Liver enzyme abnormalities [see Warnings and Precautions (5.1)]
- Diarrhea [see Warnings and Precautions (5.2)]
- Fat-Soluble Vitamin Deficiency [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
PFIC Clinical Studies
Trial 1 is a randomized, double-blind, placebo-controlled, 24-week study of two dose levels of BYLVAY (40 mcg/kg and 120 mcg/kg) administered once daily [see Clinical Studies (14.1)]. Sixty-two patients were randomized (1:1:1) to receive one of the following:
- BYLVAY 40 mcg/kg/day (n=23),
- BYLVAY 120 mcg/kg/day (n=19), or
- Placebo (n=20).
Table 5 summarizes the frequency of adverse reactions reported in ≥2% and at a rate greater than placebo in patients treated with BYLVAY in Trial 1. The most common adverse reactions observed in Trial 1 included diarrhea, liver test abnormalities, vomiting, abdominal pain, and fat-soluble vitamin deficiency.
Table 5. Common Adverse Reactions* from a Clinical Study of BYLVAY in Patients with Progressive Familial Intrahepatic Cholestasis (Trial 1)|
Adverse Reaction |
Placebo |
BYLVAY 40 mcg/kg/day |
BYLVAY 120 mcg/kg/day |
|---|---|---|---|
| |||
|
Diarrhea |
2 (10%) |
9 (39%) |
4 (21%) |
|
Transaminases increased (ALT, AST) |
1 (5%) |
3 (13%) |
4 (21%) |
|
Vomiting |
0 |
4 (17%) |
3 (16%) |
|
Abdominal pain |
0 |
3 (13%) |
3 (16%) |
|
Blood bilirubin increased |
2 (10%) |
3 (13%) |
2 (11%) |
|
Fat-soluble vitamin deficiency (A, D, E) |
1 (5%) |
0 |
3 (16%) |
|
Splenomegaly |
0 |
0 |
2 (11%) |
|
Cholelithiasis |
0 |
0 |
1 (5%) |
|
Dehydration |
0 |
0 |
1 (5%) |
|
Fracture |
0 |
1 (4%) |
0 |
Trial 2 is a 72-week, open-label, single-arm trial in PFIC type 1, 2, and 3 patients. Age of the enrolled patients ranged from 4 months to 25 years. BYLVAY 120 mcg/kg/day was administered once daily. A total of 79 PFIC patients have been enrolled, of which 56 patients were rolled over from Trial 1. In addition to patients rolled over from Trial 1, an additional 23 patients were enrolled to Trial 2. Treatment-emergent adverse reactions were similar as observed in Trial 1. The most common reason for BYLVAY treatment interruption was liver test abnormalities (increases in ALT, AST, direct and total bilirubin). Of the 12 patients who discontinued BYLVAY in Trial 2, one patient underwent biliary diversion surgery, and a second patient received liver transplantation; both underwent surgical intervention secondary to intolerable pruritus, unresponsive to BYLVAY treatment.
ALGS Clinical Studies
Trial 3 is a randomized, double-blind, placebo-controlled, 24-week study of a single dose level of BYLVAY (120 mcg/kg) administered once daily [see Clinical Studies (14.2)]. Fifty-two patients were randomized (2:1) to receive one of the following:
- BYLVAY 120 mcg/kg/day (n=35), or
- Placebo (n=17).
Table 6 summarizes the frequency of adverse reactions in patients with ALGS, reported in ≥5% and at a rate greater than placebo in patients treated with BYLVAY in Trial 3. No patients discontinued study treatment due to an adverse reaction. The most common adverse reactions observed in Trial 3 included diarrhea, abdominal pain, hematoma, and decreased weight.
Table 6. Common Adverse Reactions* from a Clinical Study of BYLVAY in Patients with Alagille Syndrome (Trial 3)|
Adverse Reaction |
Placebo |
BYLVAY 120 mcg/kg/day |
|---|---|---|
| ||
|
Diarrhea |
1 (6%) |
10 (29%) |
|
Abdominal Pain |
1 (6%) |
5 (14%) |
|
Hematoma |
0 |
3 (9%) |
|
Weight decreased |
0 |
2 (6%) |
Trial 4 is a 72-week, open-label extension study in 49 pediatric patients with ALGS aged 1 to 15 years. BYLVAY 120 mcg/kg/day was administered once daily. Treatment-emergent adverse reactions were similar to those observed in Trial 3. The most common reason for BYLVAY treatment interruption was gastrointestinal disorders, including diarrhea and abdominal pain.
PFIC: Most common adverse reactions (>2%) are liver test abnormalities, diarrhea, abdominal pain, vomiting, and fat-soluble vitamin deficiency. (6.1)
ALGS: Most common adverse reactions (>5%) are diarrhea, abdominal pain, hematoma, and decreased weight. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Albireo Pharma, Inc. at +1-855-252-4736, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Bile Acid Binding Resins
Administer bile acid binding resins (e.g., cholestyramine, colesevelam, or colestipol) at least 4 hours before or 4 hours after administration of BYLVAY [see Dosage and Administration (2.3)]. Bile acid binding resins may bind odevixibat in the gut, which may reduce BYLVAY efficacy.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Oral Pellets:
- 200 mcg: capsule with ivory opaque cap and white opaque body; imprinted "A200" (black ink).
- 600 mcg: capsule with ivory opaque cap and body; imprinted "A600" (black ink).
Capsules:
- 400 mcg: capsule with medium orange opaque cap and white opaque body; imprinted "A400" (black ink).
- 1200 mcg: capsule with medium orange opaque cap and body; imprinted "A1200" (black ink).
Oral Pellets: 200 mcg, 600 mcg (3)
Capsules: 400 mcg, 1200 mcg (3)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Odevixibat is a reversible inhibitor of the ileal bile acid transporter (IBAT). It decreases the reabsorption of bile acids (primarily the salt forms) from the terminal ileum.
Pruritus is a common symptom in patients with PFIC and ALGS; the pathophysiology of pruritus in patients with PFIC is not completely understood. Although the complete mechanism by which odevixibat improves pruritus in both PFIC and ALGS patients is unknown, it may involve inhibition of the IBAT, which results in decreased reuptake of bile salts, as observed by a decrease in serum bile acids [see Clinical Pharmacology (12.2)].
12.2 Pharmacodynamics
Odevixibat reduces serum bile acids in patients with PFIC and ALGS.
In Trial 1, a 24-week, randomized, double-blind, placebo-controlled trial conducted in 62 patients with a confirmed diagnosis of PFIC type 1 or type 2, the majority of patients (88.7%) had elevated serum bile acids above 100 mcmol/L at baseline [see Clinical Studies (14)]. Serum bile acids concentrations were reduced from baseline within 4-8 weeks of odevixibat treatment compared to placebo treatment. The decreased concentrations of serum bile acids fluctuated over time but generally were maintained during the treatment over 24 weeks. The extent of decrease in serum bile acids was similar between 40 and 120 mcg/kg.
Trial 3 is a 24-week, randomized, double-blind, placebo-controlled trial conducted in 52 patients with a confirmed diagnosis of ALGS who were administered treatment with BYLVAY 120 mcg/kg once daily [see Clinical Studies (14)]. At baseline, serum bile acids were variable ranging from 93 to 510 mcmol/L. Serum bile acid concentrations were reduced from baseline as early as Week 4 of odevixibat treatment and the reduction was generally maintained during treatment over 24 weeks.
12.3 Pharmacokinetics
In pediatric patients with PFIC, 6 months to 17 years of age who received BYLVAY 40 mcg/kg or 120 mcg/kg once daily with food in the morning, the measurable odevixibat concentrations ranged from 0.06 to 0.72 ng/mL, and odevixibat concentrations were below the limit of quantification (0.05 ng/mL) in the majority of plasma samples.
In pediatric patients with ALGS who received BYLVAY 120 mcg/kg once daily with food in the morning, the measurable odevixibat concentrations ranged from 0.05 to 3.4 ng/mL.
Following single and repeated oral administration of odevixibat from 0.1 to 3 mg in healthy adults, plasma concentrations of odevixibat were mostly below the limit of quantification (0.05 ng/mL); therefore, AUC and peak plasma concentration (Cmax) could not be calculated.
Following a single administration of odevixibat 7.2 mg in healthy adults, the mean (%CV) Cmax and AUC0-24h were 0.47 ng/mL (34.8) and 2.19 ng∙h/mL (36.2), respectively. No accumulation of odevixibat was observed following once-daily dosing.
Absorption
Odevixibat is minimally absorbed following oral administration. Following a single administration of odevixibat 7.2 mg in healthy adults, odevixibat Cmax is reached between 1 to 5 hours.
Sprinkle on Applesauce
When odevixibat 9.6 mg was administered after sprinkling the pellets on applesauce, decreases of 39% and 35% in Cmax and AUC0-24h, respectively, and delayed median Tmax from 3 hours to 4.5 hours were observed compared to administration of whole capsules (eight 1200 mcg capsules) under fasted conditions. The effect of sprinkling on soft food on systemic exposure is not clinically significant [see Dosage and Administration (2.3)].
Effect of Food
Concomitant administration of a high-fat meal (800-1000 calories with approximately 50% of total caloric content of the meal from fat) with a single dose of odevixibat 9.6 mg delayed median Tmax from 3 hours to 4.5 hours and resulted in decreases of 72% and 62% in Cmax and AUC0-24h, respectively, compared to administration under fasted conditions in healthy adults. The effect of food on the changes of systemic exposures to odevixibat is not clinically significant [see Dosage and Administration (2.3)].
Distribution
Human plasma protein binding of odevixibat is greater than 99% in vitro.
Elimination
Following a single oral dose of 7.2 mg odevixibat in healthy adults, the mean half-life (t1/2) was 2.36 hours.
Metabolism
In vitro, odevixibat was metabolized via mono-hydroxylation.
Excretion
Following a single radiolabeled odevixibat 3 mg oral dose in healthy adults, 82.9% of the dose was recovered in feces (97% unchanged) and less than 0.002% in the urine.
Drug Interaction Studies
Effect of Other Drugs on Odevixibat
Odevixibat is a substrate of P-glycoprotein (P-gp) but not a substrate of breast cancer resistance protein (BCRP).
Coadministration of itraconazole (a strong P-gp inhibitor) with a single dose of BYLVAY 7.2 mg increased odevixibat AUC0-24h by 66% and Cmax by 52%, which is not expected to have a clinically significant effect.
Effect of Odevixibat on Other Drugs
In in vitro studies, odevixibat was not an inhibitor of CYP isoforms 1A2, 2B6, 2C8, 2C9, 2C19, or 2D6 nor an inducer of CYP isoforms 1A2, 2B6, or 3A4.
Concomitant use of BYLVAY 7.2 mg once daily for 4 days with oral midazolam (a CYP3A4 substrate) in healthy adults decreased the AUC0-24h of midazolam and 1-OH midazolam by 29% and 13%, respectively, which is not expected to have a clinically relevant effect.
In in vitro studies, odevixibat did not inhibit the transporters P-gp; BCRP; organic anion transporter polypeptide 1B1 and 1B3(OATP1B1 and OATP1B3); organic anion transporter (OAT)1, OAT3; organic cation transporter 2 (OCT2), multidrug and toxin extrusion transporter 1 and 2K (MATE1 and MATE2K).
12.5 Pharmacogenomics
PFIC is a heterogenous disease caused by homozygous or compound heterozygous variants, with different PFIC subtypes occurring in the general population. PFIC2 is the most common subtype accounting for 37-90% of PFIC patients. PFIC1 is caused by variants in the aminophospholipid flippase (ATP8B1) gene, which encodes the Familial Intrahepatic Cholestasis 1 (FIC1) protein, while PFIC2 results from variants in the ABCB11 gene, which encodes the Bile Salt Export Pump (BSEP) protein. PFIC2 patients are further categorized into BSEP subgroups based on their specific variants. The BSEP-1 subgroup includes patients with at least one p.D482G (c.1445A>G) or p.E297G (c.890A>G) variant, BSEP-2 includes patients with at least one missense variant other than p.D482G or p.E297G (non BSEP-1), and BSEP-3 includes patients with variants that are predicted to encode a non-functional protein. No BSEP-3 patients were studied in clinical Trial 1 (NCT03566238). The prevalence of BSEP-1, BSEP-2, and BSEP-3 subgroups are approximately 27.3%, 51.5%, and 21.2%, respectively, based on data from a global consortium characterizing the natural history of severe BSEP deficiency.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies, odevixibat was not tumorigenic in rats or mice at oral doses up to 100 mg/kg/day. Systemic exposure to odevixibat (AUC) at the maximum dose studied in rats and mice was approximately 231 and 459 times the maximum recommended dose, respectively.
Mutagenesis
Odevixibat was negative in the in vitro bacterial reverse mutation (Ames) assay, the in vitro mouse lymphoma cell gene mutation assay, and the in vivo rat micronucleus test.
Impairment of Fertility
Odevixibat had no effects on fertility or reproductive function in male and female rats at oral doses of up to 1,000 mg/kg/day.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 PFIC
The efficacy of BYLVAY was evaluated in Trial 1 (NCT03566238), a 24-week, randomized, double-blind, placebo-controlled trial. Trial 1 was conducted in 62 pediatric patients, aged 6 months to 17 years, with a confirmed molecular diagnosis of PFIC type 1 or type 2, and presence of pruritus at baseline. Patients with variants in the ABCB11 gene that predict non-function or complete absence of the bile salt export pump (BSEP) protein, who had experienced prior hepatic decompensation events, who had other concomitant liver disease, whose INR was greater than 1.4, whose ALT or total bilirubin was greater than 10-times the upper limit of normal (ULN), or who had received a liver transplant were excluded in Trial 1.
Patients were randomized to placebo (n=20), 40 mcg/kg (n=23), or 120 mcg/kg (n=19). Study drug was administered once daily with a meal in the morning. In patients weighing less than 19.5 kg or patients who could not swallow the whole capsule, study drug was sprinkled on soft food and then administered orally.
Median age (range) of the patients in Trial 1 was 3.2 (0.5 to 15.9) years; 3 patients were older than 12 years of age. Of the 62 patients, 50% were male and 84% were white; 27% had PFIC type 1, and 73% had PFIC type 2. The mean (standard error [SE]) scratching score in the 2 weeks prior to baseline was 2.9 (0.08). Baseline mean (SE) eGFR was 164 (30.6) mL/min/1.73 m2. Baseline median (range) ALT, AST, and total bilirubin were 65 (16-798) U/L, 83.5 (32-405) U/L, and 2.2 (0.2-18.6) mg/dL, respectively.
In Trial 1, a total of 13 patients discontinued from trial prematurely either due to no improvement in pruritus (n=11) or due to adverse reactions (n=2); 5/20 (25%) patients discontinued from the placebo arm and 8/42 (19%) patients discontinued from the BYLVAY arms. A total of 11 of the 13 patients rolled over to Trial 2 to receive BYLVAY 120 mcg/kg/day. One patient treated with BYLVAY 120 mcg/kg/day withdrew from the trial due to a treatment-emergent adverse reaction of diarrhea [see Adverse Reactions (6)].
Given the patients' young age, a single-item observer-reported outcome (ObsRO) was used to measure patients' scratching severity as observed by their caregiver twice daily (once in the morning and once in the evening). Scratching severity was assessed on a 5-point ordinal response scale, with scores ranging from 0 (no scratching) to 4 (worst possible scratching). Patients were included in Trial 1 if their average scratching score was greater than or equal to 2 (medium scratching) in the 2 weeks prior to baseline.
Table 7 presents the results of the comparison between BYLVAY and placebo on the mean of patients' percentage of ObsRO assessments over the 24-week treatment period that were scored as 0 (no scratching) or 1 (a little scratching). Patients treated with BYLVAY demonstrated greater improvement in pruritus compared with placebo. Figure 1 displays the mean of patients' worst weekly average scratching scores in each treatment group for each month, where the weekly average utilized the worst score from each day (morning or evening score).
Table 7: Efficacy Results Over the 24-Week Treatment Period in Patients with PFIC Type 1 or 2 in Trial 1|
Placebo |
BYLVAY | ||
|---|---|---|---|
|
40 mcg/kg/day |
120 mcg/kg/day | ||
|
Mean* Percentage of Assessments Over the Treatment Period | |||
| |||
|
Mean (SE) |
13.2 (8.7) |
35.4 (8.1) |
30.1 (9.0) |
|
Mean Difference vs Placebo (95% CI) |
22.2 (4.7, 39.6) |
16.9 (-2.0, 35.7) |
|
|
|
14.2 ALGS
The efficacy of BYLVAY was evaluated in Trial 3 (NCT04674761), a 24-week, randomized, double-blind, placebo-controlled trial. Trial 3 was conducted in 52 pediatric patients, aged 6 months to 15 years, with a confirmed diagnosis of ALGS and presence of pruritus at baseline. Patients who had decompensated liver disease, who had other concomitant liver disease, whose INR was greater than 1.4, whose ALT was greater than 10-times the upper limit of normal (ULN) at screening, whose total bilirubin was greater than 15-times the ULN at screening, or who had received a liver transplant were excluded from Trial 3.
Patients were randomized to placebo (n=17) or 120 mcg/kg (n=35). Study drug was administered once daily with a meal in the morning. In patients weighing less than 19.5 kg or patients who could not swallow the whole capsule, study drug was sprinkled on soft food and then administered orally.
Median age (range) of the patients in Trial 3 was 6.1 (1.7 to 15.5) years in the BYLVAY group and 4.2 (0.5 to 14.3) years in the placebo group; 5 patients were older than 12 years of age. Of the 52 patients, 52% were male and 83% were white; 92% of patients had the JAG1 mutation and 8% had the NOTCH2 mutation. The mean (standard deviation [SD]) scratching score in the 2 weeks prior to baseline was 2.9 (0.6). Baseline mean (SD) eGFR was 159 (51.4) mL/min/1.73 m2. Baseline median (range) ALT, AST, and total bilirubin were 152 (39-403) U/L, 135 (57-427) U/L, and 2.0 (0.4-11.4) mg/dL, respectively.
Given the patients' young ages, a single-item observer-reported outcome (ObsRO) was used to measure patients' scratching severity as observed by their caregiver twice daily (once in the morning and once in the evening). Scratching severity was assessed on a 5-point ordinal response scale, with scores ranging from 0 (no scratching) to 4 (worst possible scratching). Patients were included in Trial 3 if the average scratching score was greater than or equal to 2 (medium scratching) in the 14 days prior to baseline.
Table 8 presents the results of the comparison between BYLVAY and placebo on the change from baseline in average scratching score based on ObsRO assessments to Month 6 (Weeks 21 to 24). The average scratching score for each patient for each month post-baseline was calculated by: (Step 1) averaging the morning scores and averaging the evening scores within a week; (Step 2) averaging the morning and evening weekly scores to yield a single weekly score; and finally (Step 3) averaging the 4 weekly scores within the month. The baseline average scratching score for each patient was calculated by averaging the weekly scores obtained in Step 2 across the 2 weeks prior to randomization and initiation of blinded treatment. Patients treated with BYLVAY demonstrated greater improvement in pruritus compared with placebo. Figure 2 displays the means (95% confidence interval) of patients' average scratching scores in each treatment group for each month.
Table 8: Efficacy Results in Patients with ALGS in Trial 3|
Placebo |
BYLVAY | |
|---|---|---|
| ||
|
Baseline Average Scratching Score | ||
|
Mean (SD) |
3.0 (0.6) |
2.8 (0.5) |
|
Change from Baseline in Average Scratching Score to Month 6 (Weeks 21 to 24)***** | ||
|
Mean (SE) |
-0.8 (0.2) |
-1.7 (0.2) |
|
Mean Difference vs Placebo (95% CI) |
-0.9 (-1.4, -0.3) | |
|
p-value |
0.002 |
|
|
Figure 2: Mean******* of the Average Scratching Scores for Each Month in Trial 3** |
|
|
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Oral Pellets
200 mcg Oral Pellets: supplied as Size 0 capsule with ivory opaque cap and white opaque body; imprinted "A200" (black ink). Supplied in bottles of 30 with child-resistant closure (NDC 74528-020-01).
600 mcg Oral Pellets: supplied as Size 0 capsule with ivory opaque cap and body; imprinted "A600" (black ink). Supplied in bottles of 30 with child- resistant closure (NDC 74528-060-01).
Capsules
400 mcg Capsule: supplied as Size 3 capsule with medium orange opaque cap and white opaque body; imprinted "A400" (black ink). Supplied in bottles of 30 with child-resistant closure (NDC 74528-040-01).
1,200 mcg Capsule: supplied as Size 3 capsule with medium orange opaque cap and body; imprinted "A1200" (black ink). Supplied in bottles of 30 with child- resistant closure (NDC 74528-120-01).
Storage and Handling:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F) [See USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Administration Instructions
Advise patients or their caregivers(s) to:
- Take BYLVAY in the morning with a meal. Do not swallow the 200 mcg or 600 mcg capsule(s) containing Oral Pellets whole. These are intended to be opened and the contents mixed into soft food or liquids [see Dosage and Administration (2.1, 2.2, 2.4)].
- Follow stepwise administration Instructions [see Dosage and Administration (2.4)] for Oral Pellets and Capsules for patients unable to swallow the capsules whole.
- Take BYLVAY at least 4 hours before or 4 hours after taking a bile acid binding resin (e.g., cholestyramine, colesevelam, or colestipol) [see Drug Interactions (7.1)].
Liver Test Abnormalities
Advise patients or their caregiver(s) that liver tests should be obtained before starting BYLVAY and periodically during BYLVAY therapy, due to the risk of elevation in liver tests and development of liver-related adverse reactions [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
Diarrhea
Advise patients or their caregiver(s) to notify their healthcare provider if they experience new onset or worsening of diarrhea [see Warnings and Precautions (5.2)].
Fat Soluble Vitamin (FSV) Deficiency
Advise patients or their caregiver(s) that INR (for vitamin K) and serum levels of vitamins A, D, E will be obtained before starting and periodically during treatment to assess for FSV deficiency [see Warnings and Precautions (5.3)].
Pregnancy
Advise patients or their caregiver(s) that there is a pregnancy safety study that collects pregnancy outcome data in women taking BYLVAY during pregnancy. Pregnant women exposed to BYLVAY, or their healthcare providers, should report BYLVAY exposure by calling 1-855-252-4736 [see Use in Specific Populations (8.1)].
INSTRUCTIONS FOR USE SECTION
Instructions For Use
BYLVAY [bil-vay]
(odevixibat)
Capsules, for oral use
Oral Pellets
This Instructions for Use contains information on how to give BYLVAY Capsules and Oral Pellets. This information does not take the place of talking to your healthcare provider about your child's medical condition or their treatment.
Important information you need to know before giving or taking BYLVAY
- Give BYLVAYalong with the morning meal.
- Mix BYLVAY in a small amount of soft food (up to 2 tablespoons [30 mL]), such as apple sauce, oatmeal, banana or carrot puree, chocolate or rice pudding, in a bowl. You may also mix BYLVAY with an age-appropriate liquid and give through an oral syringe.
- If your child is taking bile acid binding resins (for example, cholestyramine, colestipol), give them BYLVAY at least 4 hours before or 4 hours after they take the bile acid binding resin.
Preparing to Give BYLVAY
You will be provided with the number of BYLVAY Capsules or Oral Pellets prescribed by your child's healthcare provider in a child-resistant closure.
Giving BYLVAY Oral Pellets with soft food:
- The shell containing Oral Pellets are to be opened and sprinkled.Do not let your child swallow the shell containing the Oral Pellets. Dispose of (throw away) the emptied shell.
- Mix the contents of the Oral Pellets with soft food as shown inSteps 1 through 9 below.
|
Step 1. |
Give BYLVAY with the first morning meal. Place a small amount of soft food (up
to 2 tablespoons [30 mL], such as apple sauce, oatmeal, banana or carrot
puree, chocolate or rice pudding) in a bowl. Keep the soft food at, or cooler
than, room temperature. |
|
Step 2. |
Hold the shell containing Oral Pellets horizontally on both ends, twist in opposite directions and pull apart (seeFigure A). |
|
| |
|
Figure A | |
|
Step 3. |
Empty the Oral Pellets into the bowl of soft food (seeFigure B). |
|
| |
|
Figure B | |
|
Step 4. |
Gently tap the shell containing Oral Pellets to make sure that all pellets come out (seeFigure C). |
|
| |
|
Figure C | |
|
Step 5. |
If the dose requires more than 1 capsule shell, repeatStep 2 andStep 3. |
|
Step 6. |
Gently mix the Oral Pellets with a spoon into the soft food. Note that the Oral Pellets will not dissolve (seeFigure D). |
|
| |
|
Figure D | |
|
Step 7. |
Give the entire dose right away after mixing.Do not store the BYLVAY mixture for later use. |
|
Step 8. |
Give water or an age-appropriate liquid, such as breast milk or infant formula, after the dose is taken. |
|
Step 9. |
Dispose of (throw away) the empty Oral Pellet shells in the trash. |
Giving BYLVAY Oral Pellets with liquids (Using an oral dosing syringe):
- The shell containing the Oral Pellets are to be opened.
- Mix the Oral Pellets with liquid as shown inSteps 1 through 14 below.
- Dispose of (throw away) the emptied shells.Do not let your child swallow the unopened shells containing the Oral Pellets.
|
Step 1. |
Give BYLVAY with the first morning meal. |
|
Step 2. |
Hold the shell containing the Oral Pellets horizontally on both ends, twist in opposite directions and pull apart (seeFigure A). |
|
Step 3. |
Empty the Oral Pellets into a small mixing cup. Gently tap the Oral Pellet shell to ensure that all contents have been emptied into the mixing cup (see Figure E). |
|
| |
|
Step 4. |
If the dose requires more than 1 capsule shell, repeatStep 2 andStep 3. |
|
Step 5. |
Add 1 teaspoon (5 mL) of an age-appropriate liquid (for example, breast milk, infant formula, or water). |
|
Step 6. |
Let the pellets sit in the liquid for about 5 minutes to allow complete wetting. REMINDER: The pellets will not dissolve in the liquid. |
|
Step 7. |
After 5 minutes, place the tip of the oral syringe completely into the mixing cup. Pull the plunger of the syringe up slowly to withdraw the liquid and pellet mixture into the syringe. Gently push the plunger down again to expel the liquid and pellet mixture back into the mixing cup. Do this 2 to 3 times to ensure complete mixing of the pellets into the liquid. |
|
Step 8. |
Withdraw the entire contents into the syringe by pulling the plunger on the end of the syringe (seeFigure F****). |
|
| |
|
Step 9. |
Place the tip of the syringe into the front of the child's mouth between the tongue and the side of the mouth, and then gently push the plunger down to squirt the liquid and pellet mixture between the tongue and the side of the mouth. Do not squirt the liquid and pellet mixture in the back of the throat because this could cause gagging or choking (seeFigure G****). |
|
| |
|
Step 10. |
Do not give using a bottle or "sippy cup" because the Oral Pellets will not pass through the opening. The oral pellets will not dissolve in liquid. |
|
Step 11. |
Give water or an age-appropriate liquid such as breast milk or infant formula after the dose is taken. |
|
Step 12. |
Repeat Steps 8 and 9 until the entire dose has been given. |
|
Step 13. |
Check to make sure all of the liquid and pellet mixture has been swallowed. |
|
Step 14. |
Dispose of (throw away) the empty Oral Pellet shells in the trash. |
Taking BYLVAY Capsules
- Take BYLVAY Capsulesalong with your morning meal. Swallow BYLVAY Capsules whole with a glass of water.Do not chew or crush the Capsules.
- For children unable to swallow BYLVAY Capsules whole, follow instructions underPreparing to Give BYLVAY above.
How should I store BYLVAY Capsules or Oral Pellets?
Store BYLVAY at room temperature between 68°F to 77°F (20°C to 25°C).
Disposing (throwing away) of BYLVAY Capsules or Oral Pellets shells.
Dispose of (throw away) the empty BYLVAY Capsule or Oral Pellets shells in the household trash.
What are the ingredients in BYLVAY?
Active ingredient: odevixibat.
Inactive ingredients: hypromellose and microcrystalline cellulose.
Manufactured for:
Albireo Pharma, Inc.
53 State Street
19th Floor
Boston, MA 02109
This Instructions for Use has been approved by the U.S. Food and Drug
Administration.
Approved: 10/2022

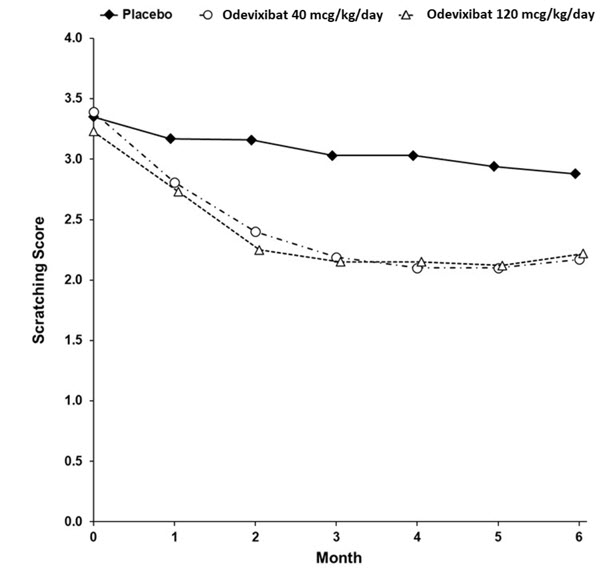
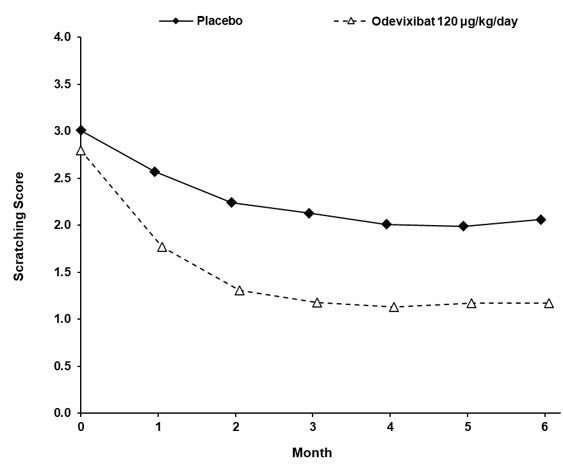
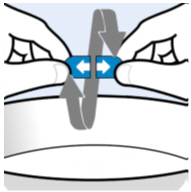
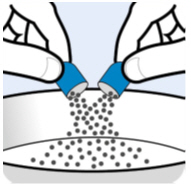
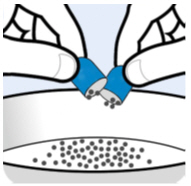
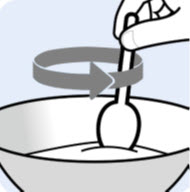
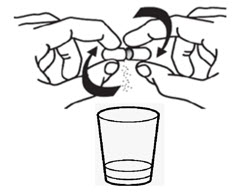 Figure E
Figure E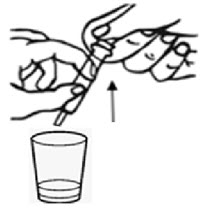 Figure F
Figure F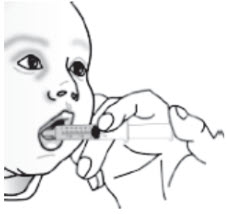 Figure G
Figure G