Methylphenidate Hydrochloride
These highlights do not include all the information needed to use METHYLPHENIDATE HYDROCHLORIDE oral solution safely and effectively. See full prescribing information for METHYLPHENIDATE HYDROCHLORIDE oral solution. METHYLPHENIDATE HYDROCHLORIDE oral solution, CII Initial U.S. Approval: 1955
298fb86d-7d94-49e1-b743-3e7801122422
HUMAN PRESCRIPTION DRUG LABEL
Nov 8, 2023
Eywa Pharma Inc
DUNS: 080465609
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Methylphenidate Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Methylphenidate Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 71930-024-52
500 mL
CII
Methylphenidate Hydrochloride Oral Solution
5 mg per 5 mL
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
eywa pharma

NDC 71930-025-52
500 mL
CII
Methylphenidate Hydrochloride Oral Solution
10 mg per 5 mL
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
eywa pharma

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
Methylphenidate hydrochloride oral solution has a high potential for abuse and misuse. The use of methylphenidate hydrochloride oral solution exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Methylphenidate hydrochloride oral solution can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride oral solution, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing methylphenidate hydrochloride oral solution, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store methylphenidate hydrochloride oral solution in a safe place, preferably locked, and instruct patients to not give methylphenidate hydrochloride oral solution to anyone else. Throughout methylphenidate hydrochloride oral solution treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid methylphenidate hydrochloride oral solution use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 bpm). Some patients may have larger increases.
Monitor all methylphenidate-treated patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-Existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought
disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Illness
CNS stimulants may induce a manic or mixed mood episode in patients. Prior to
initiating methylphenidate hydrochloride oral solution treatment, screen
patients for risk factors for developing a manic episode (e.g., comorbid or
history of depressive symptoms or a family history of suicide, bipolar
disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosages, may cause psychotic or manic
symptoms (e.g., hallucinations, delusional thinking, or mania) in patients
without a prior history of psychotic illness or mania. In a pooled analysis of
multiple short- term, placebo-controlled studies of CNS stimulants, psychotic
or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated
patients, compared to 0% in placebo-treated patients. If such symptoms occur,
consider discontinuing methylphenidate hydrochloride oral solution.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on methylphenidate, often subsequent to an increase in dosage. Priapism also occurred during methylphenidate withdrawal (drug holidays or during discontinuation).
Methylphenidate-treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, Including Raynaud’s Phenomenon
CNS stimulants, including methylphenidate hydrochloride oral solution, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in postmarketing reports and at the therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during methylphenidate hydrochloride oral solution treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for Methylphenidate-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.7 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate- treated and non-medication treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in Methylphenidate-treated pediatric patients. Pediatric patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted. The safety and effectiveness of methylphenidate hydrochloride oral solution have not been established in pediatric patient less than 6 years of age.
5.8 Acute Angle Closure Glaucoma
There have been reports of angle closure glaucoma associated with methylphenidate treatment. Although the mechanism is not clear, methylphenidate-treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
5.9 Increased Intraocular Pressure and Glaucoma
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment [see Adverse Reactions (6.2)].
Prescribe methylphenidate hydrochloride oral solution to patients with open- angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor methylphenidate-treated patients with a history of abnormally increased IOP or open-angle glaucoma.
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating methylphenidate hydrochloride oral solution, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor methylphenidate-treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
• Risks to Patients with Serious Cardiac Disease: Avoid use in patients with
known structural cardiac abnormalities, cardiomyopathy, serious cardiac
arrhythmias, coronary artery disease, or other serious cardiac disease. (5.2)
• Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse.
(5.3)
• Psychiatric Adverse Reactions: Prior to initiating methylphenidate
hydrochloride oral solution, screen patients for risk factors for developing a
manic episode. If new psychotic or manic symptoms occur, consider
discontinuing methylphenidate hydrochloride oral solution. (5.4)
• Priapism: If abnormally sustained or frequent and painful erections occur,
patients should seek immediate medical attention. (5.5)
• Peripheral Vasculopathy, Including Raynaud’s Phenomenon: Careful observation
for digital changes is necessary during methylphenidate hydrochloride oral
solution treatment. Further clinical evaluation (e.g., rheumatology referral)
may be appropriate for patients who develop signs or symptoms of peripheral
vasculopathy. (5.6)
• Long-Term Suppression of Growth in Pediatric Patients: Closely monitor
(height and weight) in pediatric patients. Pediatric patients not growing or
gaining height or weight as expected may need to have their treatment
interrupted. (5.7)
• Acute Angle Closure Glaucoma: Methylphenidate-treated patients considered at
risk for acute angle closure glaucoma (e.g., patients with significant
hyperopia) should be evaluated by an ophthalmologist. (5.8)
• Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe methylphenidate
hydrochloride oral solution to patients with open-angle glaucoma or abnormally
increased IOP only if the benefit of treatment is considered to outweigh the
risk. Closely monitor patients with a history of increased IOP or open-angle
glaucoma. (5.9)
• Motor and Verbal Tics, and Worsening of Tourette’s Syndrome: Before
initiating methylphenidate hydrochloride oral solution, assess the family
history and clinically evaluate patients for tics or Tourette’s syndrome.
Regularly monitor patients for the emergence or worsening of tics or
Tourette’s syndrome. Discontinue treatment if clinically appropriate. (5.10)
(5)
DESCRIPTION SECTION
11 DESCRIPTION
Methylphenidate hydrochloride oral solution contains methylphenidate hydrochloride a CNS stimulant. It is available as an oral solution in 5 mg/5 mL and 10 mg/5 mL strengths for oral administration. Chemically, methylphenidate hydrochloride is (d,l (racemic) methyl α-phenyl-2-piperidineacetate hydrochloride and its structural formula is:
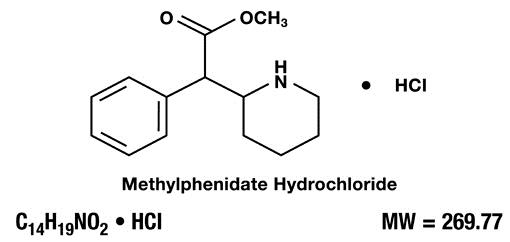
Methylphenidate hydrochloride USP is a white to off white, fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone.
Each mL of methylphenidate hydrochloride oral solution 5 mg/5 mL contains 1 mg of methylphenidate hydrochloride USP.
Each mL of methylphenidate hydrochloride oral solution 10 mg/5 mL contains 2 mg of methylphenidate hydrochloride USP.
Methylphenidate hydrochloride oral solution also contains the following inactive ingredients: glycerin, polyethylene glycol 1450, concord grape flavor N&A, diluted hydrochloric acid (10%), and purified water.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a lifetime carcinogenicity study carried out in B6C3F1 mice,
methylphenidate caused an increase in hepatocellular adenomas and, in males
only, an increase in hepatoblastomas, at a daily dose of approximately 60
mg/kg/day. This dose is approximately 5 times the maximum recommended human
dose (MRHD) of 60 mg/kg given to adults on a mg/m2 basis. Hepatoblastoma is a
relatively rare rodent malignant tumor type. There was no increase in total
malignant hepatic tumors. The mouse strain used is sensitive to the
development of hepatic tumors, and the significance of these results to humans
is unknown.
Methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 7 times the MRHD (adults) on a mg/m2 basis.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay,
in the in vitro mouse lymphoma cell forward mutation assay, or in the in vitro
chromosomal aberration assay using human lymphocytes. Sister chromatid
exchanges and chromosome aberrations were increased, indicative of a weak
clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary
(CHO) cells. Methylphenidate was negative in vivo in males and females in the
mouse bone marrow micronucleus assay.
Impairment of Fertility
No human data on the effect of methylphenidate on fertility are available.
Methylphenidate did not impair fertility in male or female mice that were fed
diets containing the drug in an 18-week continuous breeding study. The study
was conducted at doses up to 160 mg/kg/day, approximately 13 times the maximum
recommended human dose of 60 mg/day given to adults on a mg/m2 basis.
