Manufacturing Establishments (1)
Cardinal Health 107, LLC
828269675
Products (1)
Metoprolol Tartrate
55154-4745
ANDA078085
ANDA (C73584)
INTRAVENOUS
March 5, 2019
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Metoprolol tartrate Injection, USP, is a selective beta1-adrenoreceptor blocking agent, available in 5 mL vials for intravenous administration. Each vial contains a sterile solution of metoprolol tartrate USP, 5 mg, and sodium chloride USP, 45 mg. Metoprolol tartrate USP is (±)-1-(Isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol (2:1) dextro- tartrate salt, and its structural formula is:
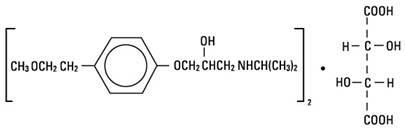
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.83. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
INDICATIONS & USAGE SECTION
Highlight: Metoprolol tartrate is a beta-adrenergic receptor inhibitor indicated for the treatment of definite or suspected acute myocardial infarction in hemodynamically stable patients to reduce cardiovascular mortality when used in conjunction with oral metoprolol maintenance therapy. (1)
1 INDICATIONS AND USAGE
Metoprolol tartrate Injection is indicated in the treatment of definite or suspected acute myocardial infarction in hemodynamically stable patients to reduce cardiovascular mortality when used in conjunction with oral metoprolol maintenance therapy.
DOSAGE & ADMINISTRATION SECTION
Highlight: •
Initiate therapy in a coronary care or similar unit immediately after the patients hemodynamic condition has stabilized. (2)
•
Begin treatment with an intravenous administration of three bolus injections of 5 mg each, at approximately 2-minute intervals. Monitor blood pressure, heart rate and electrocardiogram. (2)
•
Following administration of Metoprolol tartrate Injection, transition the patient to an oral formulation of metoprolol. (2)
2 DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Initiate treatment in a coronary care or similar unit immediately after the patient's hemodynamic condition has stabilized.
Begin treatment in this early phase with the intravenous administration of three bolus injections of 5 mg of Metoprolol tartrate each; give the injections at approximately 2-minute intervals. During the intravenous administration of Metoprolol tartrate, monitor blood pressure, heart rate, and electrocardiogram.
Transition to Oral Metoprolol:
Following administration of Metoprolol tartrate Injection, transition patients to an oral formulation of metoprolol. See prescribing information for oral metoprolol for dose selection.
DOSAGE FORMS & STRENGTHS SECTION
Highlight: Injection: 5 mg metoprolol tartrate supplied in Single-dose glass Fliptop Vial. (3)
3 DOSAGE FORMS AND STRENGTHS
Injection: 5 mg metoprolol tartrate supplied in 5 mL Single-dose glass Fliptop Vial.
CONTRAINDICATIONS SECTION
Highlight: •
Known hypersensitivity to product components. (4)
•
Severe bradycardia, greater than first degree heart block, or sick sinus syndrome without a pacemaker. (4)
•
Cardiogenic shock or decompensated heart failure. (4)
4 CONTRAINDICATIONS
Hypersensitivity to Metoprolol tartrate and related derivatives, or to any of the excipients; hypersensitivity to other beta-blockers (cross sensitivity between beta-blockers can occur).
Metoprolol tartrate is contraindicated in patients with a heart rate <45 beats/min; second- and third-degree heart block (unless a functioning pacemaker is present); significant first-degree heart block (P-R interval ≥0.24 sec); systolic blood pressure <100 mmHg; or decompensated cardiac failure.
DRUG INTERACTIONS SECTION
Highlight: •
Catecholamine-depleting drugs may have an additive effect when given with beta-blocking agents. (7.1)
•
Patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction. (7.2)
•
CYP2D6 Inhibitors are likely to increase metoprolol concentration. (7.3)
•
Concomitant use of glycosides, clonidine, and diltiazem and verapamil with beta-blockers can increase the risk of bradycardia. (7.4)
•
Beta-blockers including metoprolol, may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. (7.4)
7 DRUG INTERACTIONS
7.1 Catecholamine Depleting Drugs and Monoamine Oxidate (MAO) Inhibitors
Catecholamine depleting drugs (e.g., reserpine) and monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe patients treated with metoprolol plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
7.2 Epinephrine
While taking beta-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenge and may be unresponsive to the usual doses of epinephrine used to treat an allergic reaction.
7.3 CYP2D6 Inhibitors
Drugs that are strong inhibitors of CYP2D6, such as quinidine, fluoxetine, paroxetine, and propafenone, were shown to double metoprolol concentrations. While there is no information about moderate or weak inhibitors, these too are likely to increase metoprolol concentration. Increases in plasma concentration decrease the cardioselectivity of metoprolol [see Clinical Pharmacology (12.3)]. Monitor patients closely, when the combination cannot be avoided.
7.4 Digitalis, Clonidine, and Calcium Channel Blockers and Other Drugs that
Decrease Heart Rate
Digitalis glycosides, clonidine, diltiazem and verapamil slow atrioventricular conduction and decrease heart rate. Concomitant administration of beta- blockers with these and other drugs known to decrease heart rate such as sphingosine-1-phosphate receptor modulators (e.g. fingolimod) may result in additive heart rate lowering effects.
If clonidine and metoprolol are coadministered, withdraw the metoprolol several days before the gradual withdrawal of clonidine because beta-blockers may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. If replacing clonidine by beta-blocker therapy, delay the introduction of beta-blockers for several days after clonidine administration has stopped.
7.5 Drugs that Decrease Blood Pressure
Concomitant administration of beta-blockers with other drugs known to decrease blood pressure may result in an enhanced hypotensive effect.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
In a large (1,395 patients randomized), double-blind, placebo-controlled clinical study, Metoprolol tartrate was shown to reduce 3-month mortality by 36% in patients with suspected or definite myocardial infarction.
Patients were randomized and treated as soon as possible after their arrival in the hospital, once their clinical condition had stabilized and their hemodynamic status had been carefully evaluated. Subjects were ineligible if they had hypotension, bradycardia, peripheral signs of shock, and/or more than minimal basal rales as signs of congestive heart failure. Initial treatment consisted of intravenous followed by oral administration of Metoprolol tartrate or placebo, given in a coronary care or comparable unit. Oral maintenance therapy with Metoprolol tartrate or placebo was then continued for 3 months. After this double-blind period, all patients were given Metoprolol tartrate and followed up to 1 year.
The median delay from the onset of symptoms to the initiation of therapy was 8 hours in both the Metoprolol tartrate- and placebo-treatment groups. Among patients treated with Metoprolol tartrate, there were comparable reductions in 3-month mortality for those treated early (≤8 hours) and those in whom treatment was started later. Significant reductions in the incidence of ventricular fibrillation and in chest pain following initial intravenous therapy were also observed with Metoprolol tartrate and were independent of the interval between onset of symptoms and initiation of therapy.
In this study, patients treated with metoprolol received the drug both very early (intravenously) and during a subsequent 3-month period, while placebo patients received no beta-blocker treatment for this period. The study thus was able to show a benefit from the overall metoprolol regimen but cannot separate the benefit of very early intravenous treatment from the benefit of later beta-blocker therapy. Nonetheless, because the overall regimen showed a clear beneficial effect on survival without evidence of an early adverse effect on survival, one acceptable dosage regimen is the precise regimen used in the trial. Because the specific benefit of very early treatment remains to be defined however, it is also reasonable to administer the drug orally to patients at a later time as is recommended for certain other beta-blockers.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient's response to therapy with Metoprolol tartrate has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking Metoprolol tartrate.
SPL UNCLASSIFIED SECTION

Distributed by
Hospira, Inc., Lake Forest, IL 60045 USA
Distributed By:
Cardinal Health
Dublin, OH 43017
L49299230124
LAB-1113-3.0
