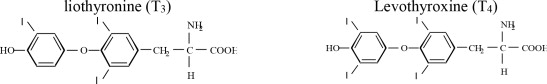Armour Thyroid
Armour Thyroid (thyroid tablets, USP)
c815faa3-1d02-4680-96ef-5fad1645e99b
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2023
PD-Rx Pharmaceuticals, Inc.
DUNS: 156893695
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
THYROID, PORCINE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Armour ® Thyroid (thyroid tablets, USP)* for oral use is a natural preparation derived from porcine thyroid glands and has a strong, characteristic odor. (T 3 liothyronine is approximately four times as potent as T 4 levothyroxine on a microgram for microgram basis.) They provide 38 mcg levothyroxine (T 4) and 9 mcg liothyronine (T 3) per grain of thyroid. The inactive ingredients are calcium stearate, dextrose, microcrystalline cellulose, sodium starch glycolate and opadry white.
|
STRUCTURAL FORMULAS |
|
|
HOW SUPPLIED SECTION
HOW SUPPLIED
Armour ® Thyroid (thyroid tablets, USP) are supplied as follows:
60 mg (1 grain) are available in bottles of 30 (NDC 55289-261-30) and bottles of 90 (NDC 55289-261-90).
Armour ® Thyroid (thyroid tablets, USP) is evenly colored, light tan, round tablets, with convex surfaces. One side is debossed with a mortar and pestle beneath the letter “A” on the top and strength code letters on the bottom as defined below
|
Strength |
Code | |
|
1 grain |
TE |
Note: (T 3 liothyronine is approximately four times as potent as T 4 levothyroxine on a microgram for microgram basis.)
Store in a tight container protected from light and moisture. Store between 15°C and 30°C (59°F and 86°F).
- Armour ® Thyroid (thyroid tablets, USP) has not been approved by FDA as a new drug.
ARMOUR ® is a registered trademark of Allergan Sales, LLC
Allergan® and its design are trademarks of Allergan, Inc.
Revised: June 2018
v.1.0USPI0457