Nitrofurantoin Monohydrate/ Macrocrystalline
Nitrofurantoin Capsules, USP (monohydrate/macrocrystals)
a4afd48b-003f-4ffa-9ca0-9432b8b0e22b
HUMAN PRESCRIPTION DRUG LABEL
Jan 4, 2024
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nitrofurantoin Monohydrate/Macrocrystalline
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Nitrofurantoin Monohydrate/ Macrocrystalline
GENERIC: Nitrofurantoin Monohydrate/Macrocrystalline
DOSAGE: CAPSULE
ADMINSTRATION: ORAL
NDC: 70518-0265-0
NDC: 70518-0265-1
NDC: 70518-0265-2
NDC: 70518-0265-3
COLOR: yellow
SHAPE: CAPSULE
SCORE: No score
SIZE: 20 mm
IMPRINT: Macrobid;52427285
PACKAGING: 30 in 1 BLISTER PACK
PACKAGING: 14 in 1 BOTTLE PLASTIC
PACKAGING: 14 in 1 BLISTER PACK
PACKAGING: 10 in 1 BOTTLE PLASTIC
ACTIVE INGREDIENT(S):
- NITROFURANTOIN MONOHYDRATE 75mg in 1
- NITROFURANTOIN 25mg in 1
INACTIVE INGREDIENT(S):
- CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED)
- STARCH, CORN
- D&C YELLOW NO. 10
- FD&C BLUE NO. 1
- FD&C RED NO. 40
- GELATIN, UNSPECIFIED
- LACTOSE, UNSPECIFIED FORM
- MAGNESIUM STEARATE
- POVIDONE, UNSPECIFIED
- TALC
- TITANIUM DIOXIDE
- SUCROSE

DESCRIPTION SECTION
DESCRIPTION
Nitrofurantoin is an antibacterial agent specific for urinary tract infections. Nitrofurantoin capsules, USP (monohydrate/macrocrystals) is a hard gelatin capsule. Each capsule contains 100 mg of nitrofurantoin in the form of 25 mg of nitrofurantoin macrocrystals and 75 mg of nitrofurantoin monohydrate.
The chemical name of nitrofurantoin macrocrystals is 1-[[[5-nitro-2-furanyl]methylene]amino]-2,4-imidazolidinedione. The chemical structure is the following:
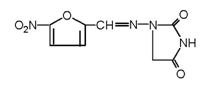
Molecular Weight: 238.16
The chemical name of nitrofurantoin monohydrate is 1-[[[5-nitro-2-furanyl]methylene]amino]-2,4- imidazolidinedione monohydrate. The chemical structure is the following:
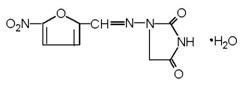
Molecular Weight: 256.17
**Inactive Ingredients:**Each capsule contains carbomer 934P, corn starch, compressible sugar, D&C Yellow No. 10, edible gray ink, FD&C Blue No. 1, FD&C Red No. 40, gelatin, lactose, magnesium stearate, povidone, talc, and titanium dioxide.
Meets USP Dissolution Test 8.
