DIVIGEL
These highlights do not include all the information needed to use DIVIGEL safely and effectively. See full prescribing information for DIVIGEL. DIVIGEL (estradiol gel), for topical use Initial U.S. Approval: 1975
59e610dc-883e-4f07-9ec6-a5841b0bf9fb
HUMAN PRESCRIPTION DRUG LABEL
May 30, 2023
Vertical Pharmaceuticals, LLC
DUNS: 173169017
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
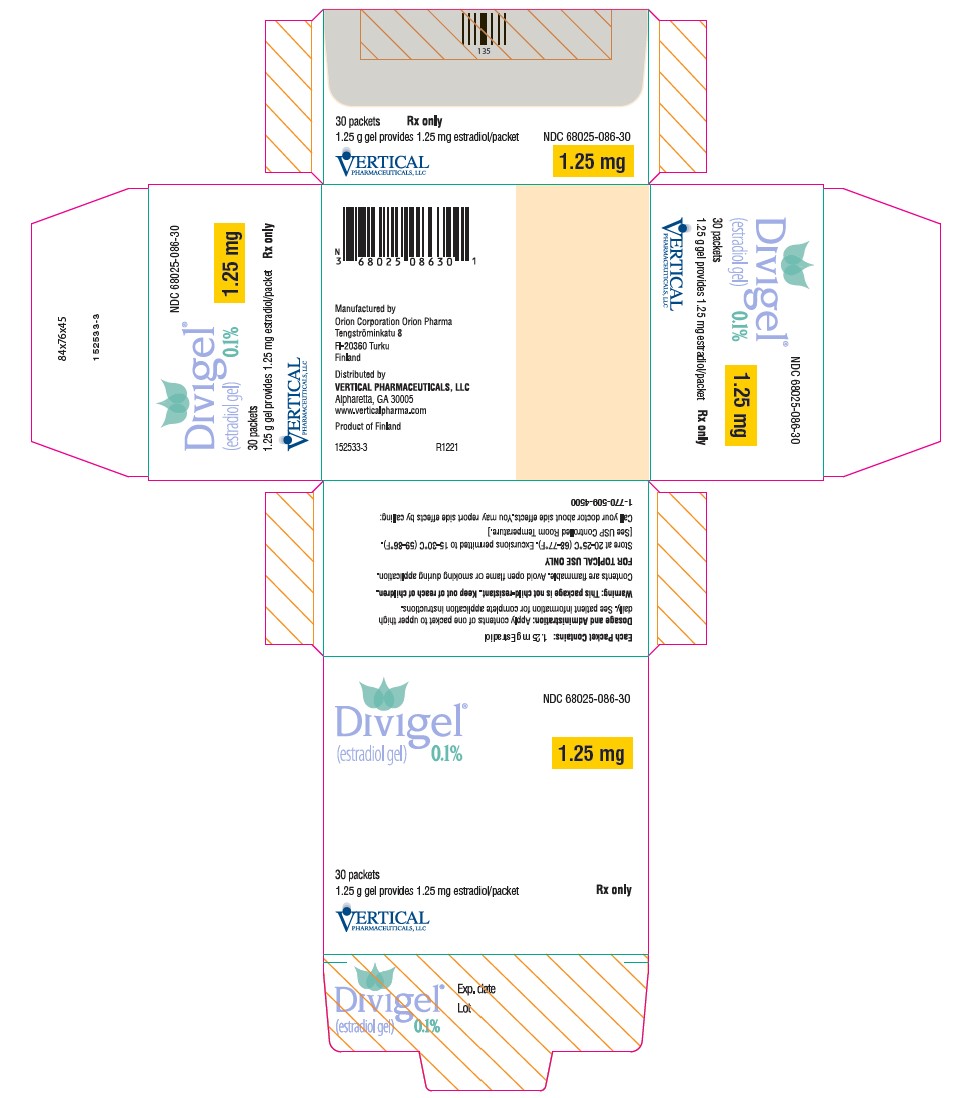
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1.25 mg Packet Carton
NDC 68025-086-30
Divigel®
(estradiol gel)****0.1%
1.25 mg
30 packets
1.25 g gel provides 1.25 mg estradiol/packet
Rx only
VERTICAL
PHARMACEUTICALS, LLC
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis and liver.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer.
Generally, a woman without a uterus, does not need a progestogen in addition to her estrogen therapy. In some cases, however, hysterectomized women who have a history of endometriosis may need a progestogen [see Warnings and Precautions (5.2, 5.14)].
Use estrogen-alone, or in combination with a progestogen, at the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Reevaluate postmenopausal women periodically as clinically appropriate to determine whether treatment is still necessary.
**2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to
Menopause**
Start therapy with the 0.25 grams applied once daily on the skin of either the right or left upper thigh. Adjust the dose up to a maximum of 1.25 grams, as needed.
The application surface area should be about 5 by 7 inches (approximately the size of two palm prints). The entire contents of a unit dose packet should be applied each day. To avoid potential skin irritation, apply Divigel to the right or left upper thigh on alternating days. Do not apply Divigel on the face, breasts, or irritated skin or in or around the vagina. Allow gel to dry after application before dressing. Do not wash the application site within 1 hour after applying Divigel. Avoid contact of the gel with eyes. Wash hands after application.
Daily administration of 0.25 to 1.25 grams of Divigel to the right or left upper thigh on alternating days. Women should be started with the lowest effective dose and the dose should be evaluated periodically (2).
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Divigel is not indicated for use in pregnant women. There are no data with the use of Divigel in pregnant women; however, epidemiologic studies and meta- analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to combined hormonal contraceptives (estrogen and progestins) before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Estrogens are present in human milk and can reduce milk production in breast- feeding women. This reduction can occur at any time but is less likely to occur once breast-feeding is well established.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Divigel and any potential adverse effects on the breastfed child from Divigel or from the underlying maternal condition.
8.4 Pediatric Use
Divigel is not indicated for use in pediatric patients. Clinical studies have not been conducted in the pediatric population.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric women involved in studies utilizing Divigel to determine whether those over 65 years of age differ from younger subjects in their response to Divigel.
The Women's Health Initiative Studies
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see Warnings and Precautions (5.1), and Clinical Studies (14.2)].
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see Warnings and Precautions (5.1, 5.2), and Clinical Studies (14.2)].
The Women's Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo [see Warnings and Precautions (5.3), and Clinical Studies (14.3)].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8 [see Warnings and Precautions (5.3), and Clinical Studies (14.3)].
DESCRIPTION SECTION
11 DESCRIPTION
Divigel (estradiol gel) 0.1 percent, is a clear, colorless gel, which is odorless when dry. It is designed to deliver sustained circulating concentrations of estradiol when applied once daily to the skin. The gel is applied to a small area (200 cm2) of the thigh in a thin layer. Divigel is available in five doses of 0.25, 0.5, 0.75, 1.0, and 1.25 grams for topical application (corresponding to 0.25, 0.5, 0.75, 1.0, and 1.25 mg estradiol, respectively).
The active component of the topical gel is estradiol.
Estradiol is a white crystalline powder, chemically described as estra-1,3,5(10)-triene-3,17ß-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.39. The structural formula is:
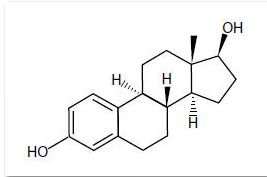
Divigel Chemical Structure
The remaining components of the gel (carbomer, ethanol, propylene glycol, purified water, and triethanolamine) are pharmacologically inactive.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, which is secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH, through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
12.2 Pharmacodynamics
Generally, a serum estrogen concentration does not predict an individual woman’s therapeutic response to Divigel nor her risk for adverse outcomes. Likewise, exposure comparisons across different estrogen products to infer efficacy or safety for the individual woman may not be valid.
12.3 Pharmacokinetics
Absorption
Estradiol diffuses across intact skin and into the systemic circulation by a passive absorption process, with diffusion across the stratum corneum being the rate-limiting factor.
In a 14-day, Phase 1, multiple-dose study, Divigel demonstrated linear and approximately dose- proportional estradiol pharmacokinetics at steady state for both AUC0-24 and Cmax following once daily dosing to the skin of either the right or left upper thigh (Table 2).
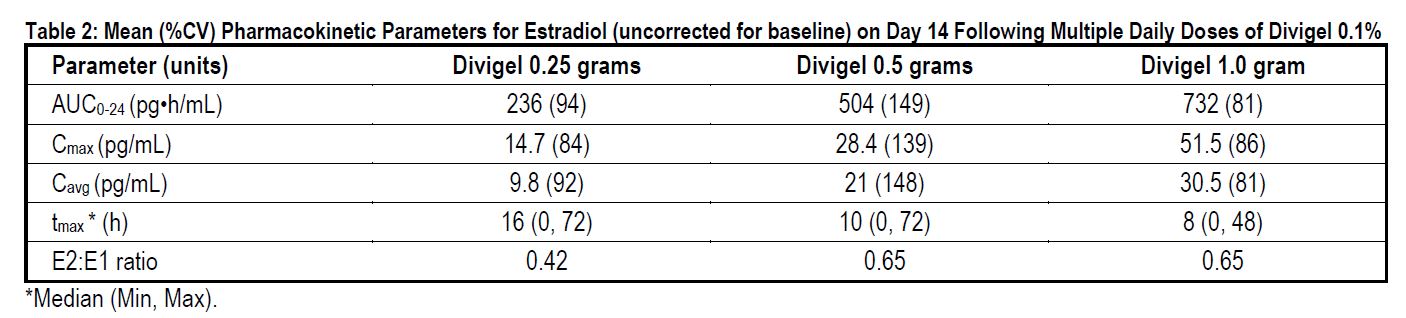
Steady-state serum concentration of estradiol are achieved by day 12 following daily application of Divigel to the skin of the upper thigh. The mean (SD) serum estradiol levels following once daily dosing at day 14 are shown in Figure 1.
Figure 1: Mean (SD) Serum Estradiol Concentrations (Values Uncorrected for Baseline) on Day 14 Following Multiple Daily Doses of Divigel 0.1%
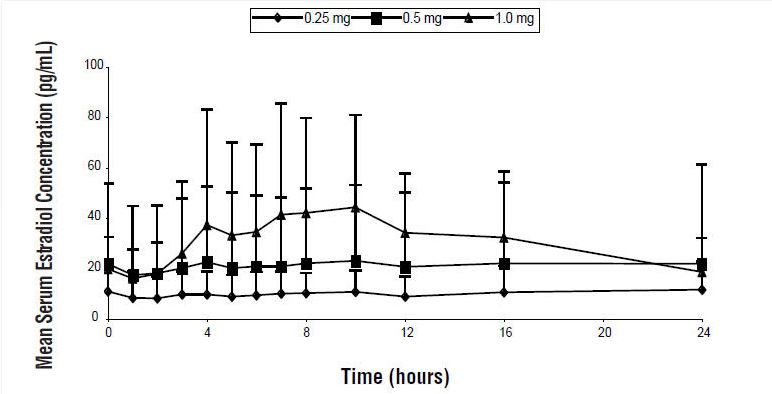
Figure 1
The effect of sunscreens and other topical lotions on the systemic exposure of Divigel has not been evaluated. Studies conducted using topical estrogen gel approved products have shown that sunscreens have the potential for changing the systemic exposure of topically applied estrogen gels.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to SHBG and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Although the clinical significance has not been determined, estradiol from Divigel does not undergo first pass metabolism and provides estradiol to estrone ratios at steady state in the range of 0.42 to 0.65.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates. The apparent terminal half-life for estradiol was about 10 hours following administration of Divigel.
Potential for Estradiol Transfer
The effect of estradiol transfer was evaluated in healthy postmenopausal women who topically applied 1.0 gram of Divigel (single dose) on one thigh. One and 8 hours after gel application, they engaged in direct thigh-to-arm contact with a partner for 15 minutes. While some elevation of estradiol levels over baseline was seen in the male subjects, the degree of transferability in this study was inconclusive.
Effects of Washing
The effect of application site washing on skin surface levels and serum concentrations of estradiol was determined in 16 healthy postmenopausal women after application of 1.0 gram of Divigel to a 200 cm2 area on the thigh. Washing the application site with soap and water 1 hour after application removed all detectable amounts of estradiol from the surface of the skin and resulted in a 30 to 38 percent decrease in the mean total 24-hour exposure to estradiol.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Divigel (estradiol gel) 0.1% is a clear, colorless, smooth, opalescent gel supplied in single-dose foil packets of 0.25, 0.5, 0.75, 1.0, and 1.25 grams, corresponding to 0.25, 0.5, 0.75, 1.0, and 1.25 mg estradiol, respectively.
NDC 68025-065-30, carton of 30 packets, 0.25 mg estradiol per single-dose foil packet
NDC 68025-066-30, carton of 30 packets, 0.5 mg estradiol per single-dose foil packet
NDC 68025-083-30, carton of 30 packets, 0.75 mg estradiol per single-dose foil packet
NDC 68025-067-30, carton of 30 packets, 1.0 mg estradiol per single-dose foil packet
NDC 68025-086-30, carton of 30 packets, 1.25 mg estradiol per single-dose foil packet
Keep out of the reach of children.
16.2 Storage and Handling
Store at 20 to 25°C (68 to 77°F). Excursions permitted to 15 to 30°C (59 to 86°F). [See USP Controlled Room Temperature.]
