Hand sanitizer
77975-016
358cb0c6-a07f-120e-e063-6294a90a167e
HUMAN OTC DRUG LABEL
May 20, 2025
Guangzhou Tingcai Cosmetic Co., Ltd.
DUNS: 529562889
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Hand sanitizer spray
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel
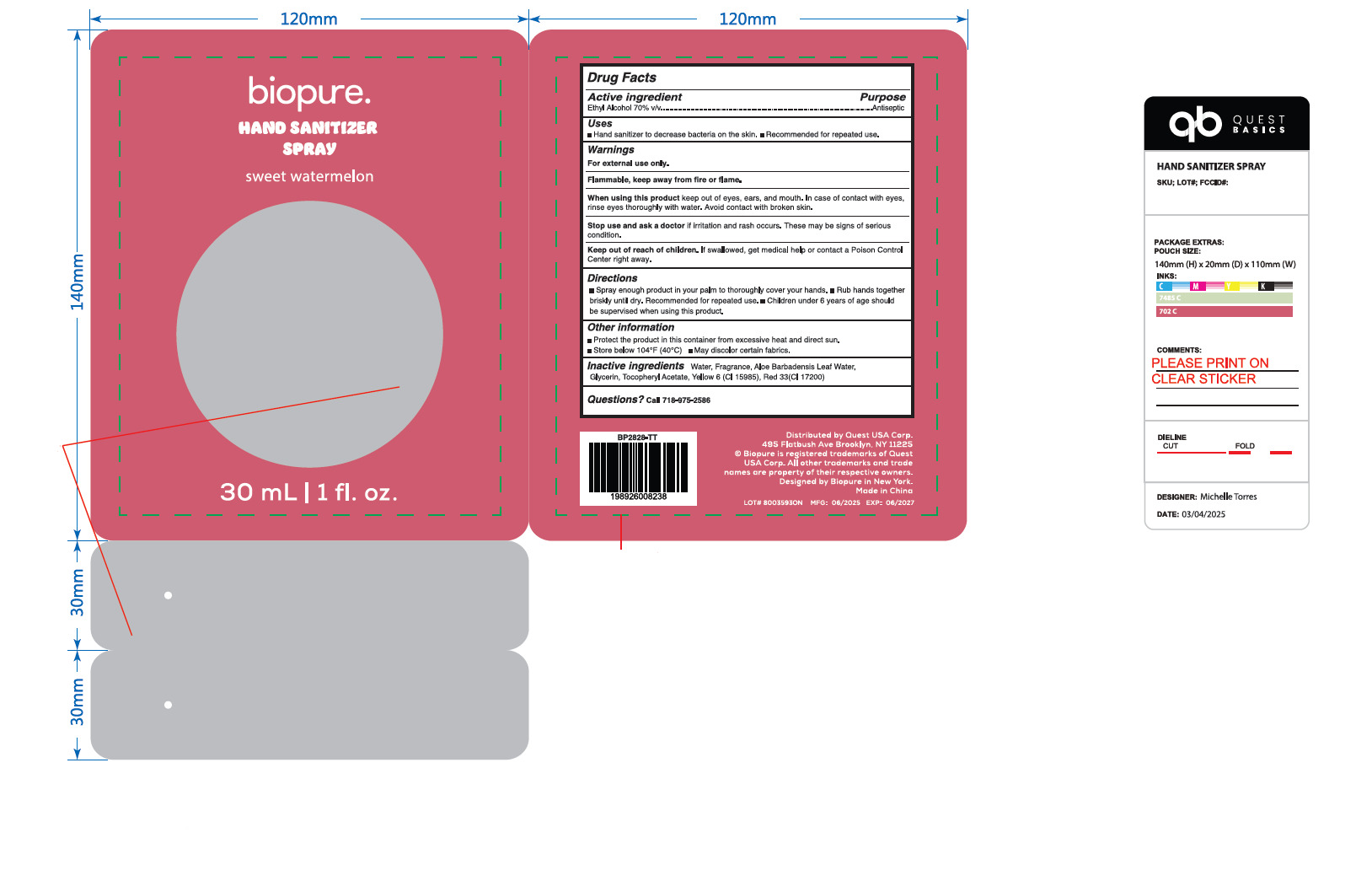
INDICATIONS & USAGE SECTION
Use
■Hand sanitizer to decrease bacteria on the skin
■Recommended for repeated use.
DOSAGE & ADMINISTRATION SECTION
Directions
■Spray enough product in your palm to thoroughly cover your hands.
■Rub hands together briskly until dry. Recommended for repeated use.
■Children under 6 years of age should be supervised when using this product.
STORAGE AND HANDLING SECTION
Other information
■Protect the product in this container from excessive heat and direct sun.
■Store below 104°F (40°C)
■ May discolor certain fabrics.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Water, Fragrance, Aloe Barbadensis Leaf Water, Glycerin, TocopheryI Acetate, Yellow 6 (CI 15985), Red 33(CI 17200)
RECENT MAJOR CHANGES SECTION
OTC - STOP USE SECTION
Stop use and ask a doctor if rritation and rash occurs. These may be signs of
serious
condition.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control
Center right away.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient(s)
ETHYL ALCOHOL 70%
OTC - PURPOSE SECTION
Purpose
Antiseptic
WARNINGS SECTION
Warnings
For external use only.
Flammable, keep away from fire or flame.
OTC - DO NOT USE SECTION
Do not use
Do not use on open skin wounds.
OTC - WHEN USING SECTION
When using this product keep out of eyes, ears, and mouth. In case of contact
with eyes,
rinse eyes thoroughly with water. Avoid contact with broken skin.
