Lopinavir and ritonavir
These highlights do not include all the information needed to use LOPINAVIR AND RITONAVIR TABLETS safely and effectively. See full prescribing information for LOPINAVIR AND RITONAVIR TABLETS. LOPINAVIR and RITONAVIR tablets, for oral use Initial U.S. Approval: 2000
4e5005b8-f00c-4671-be09-bb927190760f
HUMAN PRESCRIPTION DRUG LABEL
Jun 3, 2021
Camber Pharmaceuticals, Inc.
DUNS: 826774775
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lopinavir and ritonavir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Lopinavir and ritonavir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
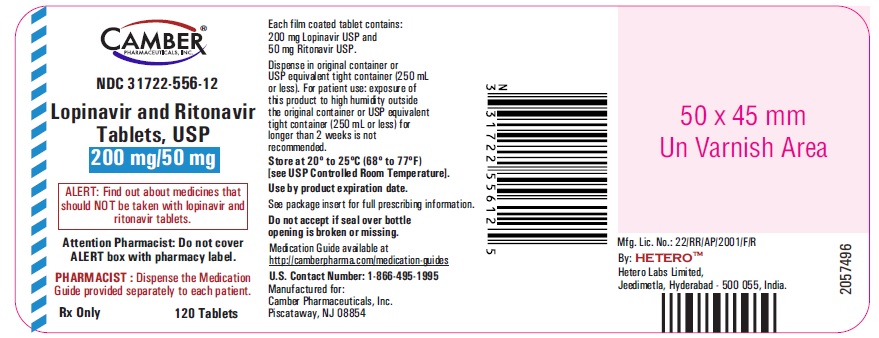
Lopinavir and Ritonavir Tablets USP, 200 mg/50 mg -Label
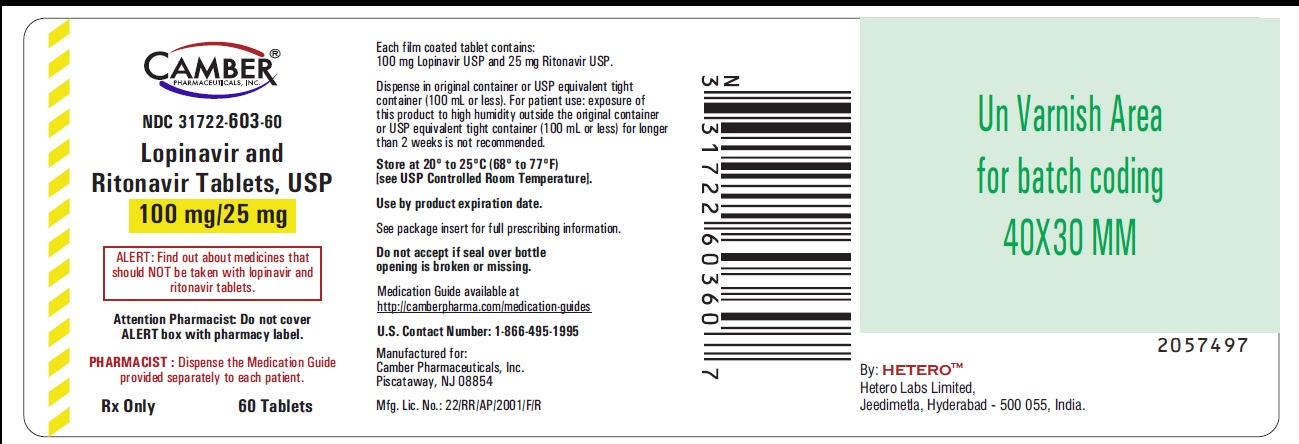
Lopinavir and Ritonavir Tablets USP, 100 mg/25 mg- Label
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Lopinavir and ritonavir tablets are indicated in combination with other
antiretroviral agents for the treatment of HIV-1 infection in adults and
pediatric patients 14 days and older.
Limitations of Use:
• Genotypic or phenotypic testing and/or treatment history should guide the
use of lopinavir and ritonavir tablets. The number of baseline lopinavir
resistance-associated substitutions affects the virologic response to
lopinavir and ritonavir tablets [see Microbiology (12.4)].
Lopinavir and ritonavir tablets are an HIV-1 protease inhibitor indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients (14 days and older). (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
• Lopinavir and ritonavir tablets are contraindicated in patients with
previously demonstrated clinically significant hypersensitivity (e.g., toxic
epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme,
urticaria, angioedema) to any of its ingredients, including ritonavir. •
Lopinavir and ritonavir tablets are contraindicated with drugs that are highly
dependent on CYP3A for clearance and for which elevated plasma concentrations
are associated with serious and/or life-threatening reactions o Alpha
1-Adrenoreceptor Antagonist: alfuzosin o Antianginal: ranolazine o
Antiarrhythmic: dronedarone o Anti-gout: colchicine o Antipsychotics:
lurasidone, pimozide o Ergot Derivatives: dihydroergotamine, ergotamine,
methylergonovine o GI Motility Agent: cisapride o Hepatitis C direct acting
antiviral: elbasvir/grazoprevir o HMG-CoA Reductase Inhibitors: lovastatin,
simvastatin o triglyceride transfer protein (MTTP) Inhibitor: lomitapide o
PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary
arterial hypertension o Sedative/Hypnotics: triazolam, orally administered
midazolam • Lopinavir and ritonavir tablets are contraindicated with drugs
that are potent CYP3A inducers where significantly reduced lopinavir plasma
concentrations may be associated with the potential for loss of virologic
response and possible resistance and cross-resistance o Anticancer Agents:
apalutamide o Antimycobacterial: rifampin o Herbal Products: St. John's Wort
(hypericum perforatum) • Lopinavir and ritonavir tablets are contraindicated
in patients with previously demonstrated clinically significant
hypersensitivity (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome,
erythema multiforme, urticaria, angioedema) to any of its ingredients,
including ritonavir.
• Lopinavir and ritonavir tablets are contraindicated with drugs that are
highly dependent on CYP3A for clearance and for which elevated plasma
concentrations are associated with serious and/or life-threatening reactions
[see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
o Alpha 1-Adrenoreceptor Antagonist: alfuzosin
o Antianginal: ranolazine
o Antiarrhythmic: dronedarone
o Anti-gout: colchicine
o Antipsychotics: lurasidone, pimozide
o Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
o GI Motility Agent: cisapride
o Hepatitis C direct acting antiviral: elbasvir/grazoprevir
o HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
o Microsomal triglyceride transfer protein (MTTP) Inhibitor: lomitapide
o PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of
pulmonary arterial hypertension
o Sedative/Hypnotics: triazolam, orally administered midazolam
• Lopinavir and ritonavir tablets are contraindicated with drugs that are
potent CYP3A inducers where significantly reduced lopinavir plasma
concentrations may be associated with the potential for loss of virologic
response and possible resistance and cross-resistance [see Drug Interactions (7.2)and Clinical Pharmacology (12.3)].
o Anticancer Agents: apalutamide
o Antimycobacterial: rifampin
o Herbal Products: St. John's Wort (hypericum perforatum)
• Hypersensitivity to lopinavir and ritonavir tablets (e.g., toxic epidermal
necrolysis, Stevens-Johnson syndrome, erythema multiforme, urticaria,
angioedema) or any of its ingredients, including ritonavir. (4)
• Co-administration with drugs highly dependent on CYP3A for clearance and for
which elevated plasma levels may result in serious and/or life-threatening
events. (4)
• Co-administration with potent CYP3A inducers where significantly reduced
lopinavir plasma concentrations may be associated with the potential for loss
of virologic response and possible resistance and cross resistance. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other
sections of the labeling.
• QT Interval Prolongation, PR Interval Prolongation [see Warnings and Precautions ( 5.5, 5.6)]
• Drug Interactions [see Warnings and Precautions (5.1)]
• Pancreatitis [see Warnings and Precautions (5.3)]
• Hepatotoxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse
reactions rates observed in the clinical trials of a drug cannot be directly
compared to rates in the clinical trials of another drug and may not reflect
the rates observed in clinical practice.
Adverse Reactions in Adults
The safety of lopinavir and ritonavir has been investigated in about 2,600
patients in Phase II-IV clinical trials, of which about 700 have received a
dose of 800/200 mg (6 capsules or 4 tablets) once daily. Along with nucleoside
reverse transcriptase inhibitors (NRTIs), in some studies, lopinavir and
ritonavir was used in combination with efavirenz or nevirapine.
In clinical studies the incidence of diarrhea in patients treated with either
lopinavir and ritonavir capsules or tablets was greater in those patients
treated once daily than in those patients treated twice daily. Any grade of
diarrhea was reported by at least half of patients taking once daily lopinavir
and ritonavir capsules or tablets. At the time of treatment discontinuation,
4.2 to 6.3% of patients taking once daily lopinavir and ritonavir and 1.8 to
3.7% of those taking twice daily lopinavir and ritonavir reported ongoing
diarrhea.
Commonly reported adverse reactions to lopinavir and ritonavir included
diarrhea, nausea, vomiting, hypertriglyceridemia and hypercholesterolemia.
Diarrhea, nausea and vomiting may occur at the beginning of the treatment
while hypertriglyceridemia and hypercholesterolemia may occur later. The
following have been identified as adverse reactions of moderate or severe
intensity (Table 8):
Table 8. Adverse Reactions of Moderate or Severe Intensity Occurring in at Least 0.1% of Adult Patients Receiving Lopinavir and Ritonavir in Combined Phase II/IV Studies (N=2,612)
|
System Organ Class (SOC) and Adverse Reaction |
n |
% |
|
BLOOD AND LYMPHATIC SYSTEM DISORDERS | ||
|
anemia* |
54 |
2.1 |
|
leukopenia and neutropenia* |
44 |
1.7 |
|
lymphadenopathy* |
35 |
1.3 |
|
CARDIAC DISORDERS | ||
|
atherosclerosis such as myocardial infarction* |
10 |
0.4 |
|
atrioventricular block* |
3 |
0.1 |
|
tricuspid valve incompetence* |
3 |
0.1 |
|
EAR****AND LABYRINTH DISORDERS | ||
|
vertigo* |
7 |
0.3 |
|
Tinnitus |
6 |
0.2 |
|
ENDOCRINE DISORDERS | ||
|
hypogonadism* |
16 |
0.81 |
|
EYE****DISORDERS | ||
|
visual impairment* |
8 |
0.3 |
|
GASTROINTESTINAL DISORDERS | ||
|
diarrhea* |
510 |
19.5 |
|
Nausea |
269 |
10.3 |
|
vomiting* |
177 |
6.8 |
|
abdominal pain (upper and lower)* |
160 |
6.1 |
|
gastroenteritis and colitis* |
66 |
2.5 |
|
dyspepsia |
53 |
2.0 |
|
pancreatitis* |
45 |
1.7 |
|
Gastroesophageal Reflux Disease (GERD)* |
40 |
1.5 |
|
hemorrhoids |
39 |
1.5 |
|
flatulence |
36 |
1.4 |
|
abdominal distension |
34 |
1.3 |
|
constipation* |
26 |
1.0 |
|
stomatitis and oral ulcers* |
24 |
0.9 |
|
duodenitis and gastritis* |
20 |
0.8 |
|
gastrointestinal hemorrhage including rectal hemorrhage* |
13 |
0.5 |
|
dry mouth |
9 |
0.3 |
|
gastrointestinal ulcer* |
6 |
0.2 |
|
fecal incontinence |
5 |
0.2 |
|
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS | ||
|
fatigue including asthenia* |
198 |
7.6 |
|
HEPATOBILIARY DISORDERS | ||
|
hepatitis including AST, ALT, and GGT increases* |
91 |
3.5 |
|
hepatomegaly |
5 |
0.2 |
|
cholangitis |
3 |
0.1 |
|
hepatic steatosis |
3 |
0.1 |
|
IMMUNE SYSTEM DISORDERS | ||
|
hypersensitivity including urticaria and angioedema* |
70 |
2.7 |
|
immune reconstitution syndrome |
3 |
0.1 |
|
INFECTIONS AND INFESTATIONS | ||
|
upper respiratory tract infection* |
363 |
13.9 |
|
lower respiratory tract infection* |
202 |
7.7 |
|
skin infections including cellulitis, folliculitis, and furuncle* |
86 |
3.3 |
|
METABOLISM AND NUTRITION DISORDERS | ||
|
hypercholesterolemia* |
192 |
7.4 |
|
hypertriglyceridemia* |
161 |
6.2 |
|
weight decreased* |
61 |
2.3 |
|
decreased appetite |
52 |
2.0 |
|
blood glucose disorders including diabetes mellitus* |
30 |
1.1 |
|
weight increased* |
20 |
0.8 |
|
lactic acidosis* |
11 |
0.4 |
|
increased appetite |
5 |
0.2 |
|
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS | ||
|
musculoskeletal pain including arthralgia and back pain* |
166 |
6.4 |
|
myalgia* |
46 |
1.8 |
|
muscle disorders such as weakness and spasms* |
34 |
1.3 |
|
rhabdomyolysis* |
18 |
0.7 |
|
osteonecrosis |
3 |
0.1 |
|
NERVOUS SYSTEM DISORDERS | ||
|
headache including migraine* |
165 |
6.3 |
|
insomnia* |
99 |
3.8 |
|
neuropathy and peripheral neuropathy* |
51 |
2.0 |
|
dizziness* |
45 |
1.7 |
|
ageusia* |
19 |
0.7 |
|
convulsion* |
9 |
0.3 |
|
tremor* |
9 |
0.3 |
|
cerebral vascular event* |
6 |
0.2 |
|
PSYCHIATRIC****DISORDERS | ||
|
anxiety* |
101 |
3.9 |
|
abnormal dreams* |
19 |
0.7 |
|
libido decreased |
19 |
0.7 |
|
RENAL AND URINARY DISORDERS | ||
|
renal failure* |
31 |
1.2 |
|
hematuria* |
20 |
0.8 |
|
nephritis* |
3 |
0.1 |
|
REPRODUCTIVE SYSTEM AND BREAST DISORDERS | ||
|
9 |
34 |
1.71 |
|
menstrual disorders - amenorrhea, menorrhagia* |
10 |
1.72 |
|
SKIN****AND SUBCUTANEOUS TISSUE DISORDERS | ||
|
rash including maculopapular rash* |
99 |
3.8 |
|
lipodystrophy acquired including facial wasting* |
58 |
2.2 |
|
dermatitis/rash including eczema and seborrheic dermatitis* |
50 |
1.9 |
|
night sweats* |
42 |
1.6 |
|
pruritus* |
29 |
1.1 |
|
alopecia |
10 |
0.4 |
|
capillaritis and vasculitis* |
3 |
0.1 |
|
VASCULAR DISORDERS | ||
|
hypertension* |
47 |
1.8 |
|
deep vein thrombosis* |
17 |
0.7 |
|
*Represents a medical concept including several similar MedDRA PTs |
Laboratory Abnormalities in Adults
The percentages of adult patients treated with combination therapy with Grade 3 to 4 laboratory abnormalities are presented in Table 9 (treatment-naïve patients) and Table 10 (treatment-experienced patients).
Table 9. Grade 3 to 4 Laboratory Abnormalities Reported in ³ 2% of Adult Antiretroviral-Naïve Patients
|
Study 863 |
Study 720 |
Study 730 | ||||
|
Variable |
Limit****1 |
Lopinavir and Ritonavir 400/100 mg Twice Daily + d4T +3TC |
Nelfinavir 750 mg |
Lopinavir |
Lopinavir |
Lopinavir |
|
Chemistry |
High | |||||
|
Glucose |
|
2% |
2% |
4% |
0% |
<1% |
|
Uric Acid |
|
2% |
2% |
5% |
<1% |
1% |
|
SGOT/AST 2 |
|
2% |
4% |
10% |
1% |
2% |
|
SGPT/ALT 2 |
|
4% |
4% |
11% |
1% |
1% |
|
GGT |
|
N/A |
N/A |
10% |
N/A |
N/A |
|
Total Cholesterol |
|
9% |
5% |
27% |
4% |
3% |
|
Triglycerides |
|
9% |
1% |
29% |
3% |
6% |
|
Amylase |
|
3% |
2% |
4% |
N/A |
N/A |
|
Lipase |
|
N/A |
N/A |
N/A |
3% |
5% |
|
Chemistry |
Low | |||||
|
Calculated Creatinine Clearance |
<50 mL/min |
N/A |
N/A |
N/A |
2% |
2% |
|
Hematology |
Low | |||||
|
Neutrophils |
<0.75 x 10 9/L |
1% |
3% |
5% |
2% |
1% |
|
1 ULN = upper limit of the normal range; N/A = Not Applicable. |
Table 10. Grade 3 to 4 Laboratory Abnormalities Reported in ³ 2% of Adult Protease Inhibitor-Experienced Patients
|
Study 888 |
Study 9572 and |
Study 802 | ||||
|
Variable |
Limit****1 |
Lopinavir |
Investigator- Selected Protease Inhibitor(s) + NVP + NRTIs |
Lopinavir and |
Lopinavir and Ritonavir |
Lopinavir and Ritonavir |
|
Chemistry |
High | |||||
|
Glucose |
|
1% |
2% |
5% |
2% |
2% |
|
Total Bilirubin |
|
1% |
3% |
1% |
1% |
1% |
|
SGOT/AST 4 |
|
5% |
11% |
8% |
3% |
2% |
|
SGPT/ALT 4 |
|
6% |
13% |
10% |
2% |
2% |
|
GGT |
|
N/A |
N/A |
29% |
N/A |
N/A |
|
Total Cholesterol |
|
20% |
21% |
39% |
6% |
7% |
|
Triglycerides |
|
25% |
21% |
36% |
5% |
6% |
|
Amylase |
|
4% |
8% |
8% |
4% |
4% |
|
Lipase |
|
N/A |
N/A |
N/A |
4% |
1% |
|
Creatine Phosphokinase |
|
N/A |
N/A |
N/A |
4% |
5% |
|
Chemistry |
Low | |||||
|
Calculated Creatinine Clearance |
<50 mL/min |
N/A |
N/A |
N/A |
3% |
3% |
|
Inorganic Phosphorus |
<1.5 mg/dL |
1% |
0% |
2% |
1% |
<1% |
|
Hematology |
Low | |||||
|
Neutrophils |
<0.75 x 10 9/L |
1% |
2% |
4% |
3% |
4% |
|
Hemoglobin |
<80 g/L |
1% |
1% |
1% |
1% |
2% |
|
1 ULN = upper limit of the normal range; N/A = Not Applicable. |
Adverse Reactions in Pediatric Patients
Lopinavir and ritonavir oral solution dosed up to 300/75 mg/m2 has been
studied in 100 pediatric patients 6 months to 12 years of age. The adverse
reaction profile seen during Study 940 was similar to that for adult patients.
Dysgeusia (22%), vomiting (21%), and diarrhea (12%) were the most common
adverse reactions of any severity reported in pediatric patients treated with
combination therapy for up to 48 weeks in Study 940. A total of 8 patients
experienced adverse reactions of moderate to severe intensity. The adverse
reactions meeting these criteria and reported for the 8 subjects include:
hypersensitivity (characterized by fever, rash and jaundice), pyrexia, viral
infection, constipation, hepatomegaly, pancreatitis, vomiting, alanine
aminotransferase increased, dry skin, rash, and dysgeusia. Rash was the only
event of those listed that occurred in 2 or more subjects (N = 3).
Lopinavir and ritonavir oral solution dosed at 300/75 mg/m 2 has been studied
in 31 pediatric patients 14 days to 6 months of age. The adverse reaction
profile in Study 1030 was similar to that observed in older children and
adults. No adverse reaction was reported in greater than 10% of subjects.
Adverse drug reactions of moderate to severe intensity occurring in 2 or more
subjects included decreased neutrophil count (N=3), anemia (N=2), high
potassium (N=2), and low sodium (N=2).
Lopinavir and ritonavir oral solution and soft gelatin capsules dosed at
higher than recommended doses including 400/100 mg/m 2 (without concomitant
NNRTI) and 480/120 mg/m 2 (with concomitant NNRTI) have been studied in 26
pediatric patients 7 to 18 years of age in Study 1038. Patients also had
saquinavir mesylate added to their regimen at Week 4. Rash (12%), blood
cholesterol abnormal (12%) and blood triglycerides abnormal (12%) were the
only adverse reactions reported in greater than 10% of subjects. Adverse drug
reactions of moderate to severe intensity occurring in 2 or more subjects
included rash (N=3), blood triglycerides abnormal (N=3), and electrocardiogram
QT prolonged (N=2). Both subjects with QT prolongation had additional
predisposing conditions such as electrolyte abnormalities, concomitant
medications, or pre-existing cardiac abnormalities.
Laboratory Abnormalities in Pediatric Patients
The percentages of pediatric patients treated with combination therapy including lopinavir and ritonavir with Grade 3 to 4 laboratory abnormalities are presented in Table 11.
Table 11. Grade 3 to 4 Laboratory Abnormalities Reported in ≥ 2% Pediatric Patients in Study 940
|
Variable |
Limit****1 |
Lopinavir and Ritonavir Twice Daily + RTIs |
|
Chemistry |
High | |
|
Sodium |
|
3% |
|
Total Bilirubin |
³ 3.0 x ULN |
3% |
|
SGOT/AST |
|
8% |
|
SGPT/ALT |
|
7% |
|
Total Cholesterol |
|
3% |
|
Amylase |
|
7% 2 |
|
Chemistry |
Low | |
|
Sodium |
< 130 mEq/L |
3% |
|
Hematology |
Low | |
|
Platelet Count |
< 50 x 10 9/L |
4% |
|
Neutrophils |
< 0.40 x 10 9/L |
2% |
|
1ULN = upper limit of the normal range. |
6.2 Postmarketing Experience
The following adverse reactions have been reported during postmarketing use of lopinavir and ritonavir. Because these reactions are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to lopinavir and ritonavir exposure.
Body as a Whole
Redistribution/accumulation of body fat has been reported [see Warnings and Precautions (5.10)].
Cardiovascular
Bradyarrhythmias. First-degree AV block, second-degree AV block, third-degree AV block, QTc interval prolongation, torsades (torsade) de pointes [see Warnings and Precautions ( 5.5,5.6)].
Renal and Urinary Disorders
Nephrolithiasis
Skin and Appendages
Toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome and erythema multiforme.
Commonly reported adverse reactions to lopinavir and ritonavir included
diarrhea, nausea, vomiting, hypertriglyceridemia and hypercholesterolemia.
(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at
1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Potential for Lopinavir and Ritonavir to Affect Other Drugs
Lopinavir/ritonavir is an inhibitor of CYP3A and may increase plasma
concentrations of agents that are primarily metabolized by CYP3A. Agents that
are extensively metabolized by CYP3A and have high first pass metabolism
appear to be the most susceptible to large increases in AUC (> 3-fold) when
co-administered with lopinavir and ritonavir. Thus, co-administration of
lopinavir and ritonavir with drugs highly dependent on CYP3A for clearance and
for which elevated plasma concentrations are associated with serious and/or
life-threatening events is contraindicated. Co-administration with other CYP3A
substrates may require a dose adjustment or additional monitoring as shown in
Table 12.
Additionally, lopinavir and ritonavir induces glucuronidation.
Published data suggest that lopinavir is an inhibitor of OATP1B1.
These examples are a guide and not considered a comprehensive list of all
possible drugs that may interact with lopinavir/ritonavir. The healthcare
provider should consult appropriate references for comprehensive information.
7.2 Potential for Other Drugs to Affect Lopinavir
Lopinavir/ritonavir is a CYP3A substrate; therefore, drugs that induce CYP3A may decrease lopinavir plasma concentrations and reduce lopinavir and ritonavir’s therapeutic effect. Although not observed in the lopinavir and ritonavir/ketoconazole drug interaction study, co-administration of lopinavir and ritonavir and other drugs that inhibit CYP3A may increase lopinavir plasma concentrations.
7.3 Established and Other Potentially Significant Drug Interactions
Table 12 provides a listing of established or potentially clinically
significant drug interactions. Alteration in dose or regimen may be
recommended based on drug interaction studies or predicted interaction [see Contraindications ( 4), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]for magnitude of interaction.
Table 12. Established and Other Potentially Significant Drug Interactions
|
Concomitant Drug Class: Drug Name |
Effect on Concentration of Lopinavir or Concomitant Drug |
Clinical Comments |
|---|---|---|
|
HIV-1 Antiviral Agents | ||
|
HIV-1 Protease Inhibitor: fosamprenavir/ritonavir |
↓ amprenavir |
An increased rate of adverse reactions has been observed with co- administration of these medications. Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV-1 Protease Inhibitor: indinavir* |
↑ indinavir |
Decrease indinavir dose to 600 mg twice daily, when co administered with lopinavir and ritonavir400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with indinavir. |
|
HIV-1 Protease Inhibitor: nelfinavir* |
↑ nelfinavir |
Lopinavir and ritonavir once daily in combination with nelfinavir is not recommended [see Dosage and Administration (2)]. |
|
HIV-1 Protease Inhibitor: ritonavir* |
↑ lopinavir |
Appropriate doses of additional ritonavir in combination with lopinavir and ritonavirwith respect to safety and efficacy have not been established. |
|
HIV-1 Protease Inhibitor: saquinavir |
↑ saquinavir |
The saquinavir dose is 1000 mg twice daily, when co administered with lopinavir and ritonavir 400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with saquinavir. |
|
HIV-1 Protease Inhibitor: tipranavir* |
↓ lopinavir |
Co-administration with tipranavir (500 mg twice daily) and ritonavir (200 mg twice daily) is not recommended. |
|
HIV CCR5 - Antagonist: maraviroc* |
↑ maraviroc |
When co-administered, patients should receive 150 mg twice daily of maraviroc. For further details see complete prescribing information for maraviroc. |
|
Non-nucleoside Reverse Transcriptase Inhibitors: efavirenz*, |
↓lopinavir |
Increase the dose of lopinavir and ritonavir tablets to 500/125 mg when lopinavir and ritonavir tablet is co-administered with efavirenz or nevirapine. Lopinavir and ritonavir tablets once daily in combination with efavirenz or nevirapine is not recommended [see Dosage and Administration (2)]. |
|
Non-nucleoside Reverse |
↑ lopinavir |
Appropriate doses of the combination with respect to safety and efficacy have not been established. |
|
Nucleoside Reverse |
Lopinavir and ritonavir tablets can be administered simultaneously with
didanosine without food. | |
|
Nucleoside Reverse |
↑ tenofovir |
Patients receiving lopinavir and ritonavir and tenofovir should be monitored for adverse reactions associated with tenofovir. |
|
Nucleoside Reverse |
↓ abacavir |
The clinical significance of this potential interaction is unknown. |
|
Other Agents | ||
|
Alpha 1-Adrenoreceptor Antagonist: alfuzosin |
↑ alfuzosin |
Contraindicated due to potential hypotension [see Contraindications (4)]. |
|
Antianginal: ranolazine |
↑ ranolazine |
Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
|
Antiarrhythmics: dronedarone |
↑ dronedarone |
Contraindicated due to potential for cardiac arrhythmias [see Contraindications (4)]. |
|
Antiarrhythmics e.g. |
↑ antiarrhythmics |
Caution is warranted and therapeutic concentration monitoring (if available) is recommended for antiarrhythmics when co-administered with lopinavir and ritonavir. |
|
Anticancer Agents: |
↑ anticancer agents |
Apalutamide is contraindicated due to potential for loss of virologic response
and possible resistance to lopinavir and ritonavir or to the class of protease
inhibitors [see Contraindications (4)]. |
|
Anticoagulants: rivaroxaban |
↑↓ warfarin ↑ rivaroxaban |
Concentrations of warfarin may be affected. Initial frequent monitoring of the
INR during lopinavir and ritonavir and warfarin co-administration is
recommended. |
|
Anticonvulsants: |
↓ lopinavir |
Lopinavir and ritonavir may be less effective due to decreased lopinavir
plasma concentrations in patients taking these agents concomitantly and should
be used with caution. |
|
Anticonvulsants: |
↓ lamotrigine |
A dose increase of lamotrigine or valproate may be needed when coadministered with lopinavir and ritonavirand therapeutic concentration monitoring for lamotrigine may be indicated; particularly during dosage adjustments. |
|
Antidepressant: |
↓ bupropion |
Patients receiving lopinavir and ritonavir and bupropion concurrently should be monitored for an adequate clinical response to bupropion. |
|
Antidepressant: |
↑ trazodone |
Adverse reactions of nausea, dizziness, hypotension and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. |
|
Anti-infective: |
↑ clarithromycin |
For patients with renal impairment, adjust clarithromycin dose as follows:
No dose adjustment for patients with normal renal function is necessary. |
|
Antifungals: |
↑ ketoconazole |
High doses of ketoconazole (>200 mg/day) or itraconazole (> 200 mg/day) are not recommended. The coadministration of voriconazole and lopinavir and ritonavir should be avoided unless an assessment of the benefit/risk to the patient justifies the use of voriconazole. Isavuconazonium and lopinavir and ritonavir should be coadministered with caution. Alternative antifungal therapies should be considered in these patients. |
|
Anti-gout: |
↑ colchicine |
Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see Contraindications (4)]. For patients with normal renal or hepatic function: Treatment of gout flares-co-administration of colchicine in patients on
lopinavir and ritonavir: Prophylaxis of gout flares-co-administration of colchicine in patients on
lopinavir and ritonavir: Treatment of familial Mediterranean fever (FMF)-co-administration of
colchicine in patients on lopinavir and ritonavir: |
|
Antimycobacterial: |
↓ lopinavir |
Contraindicated due to potential loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors or other co-administered antiretroviral agents [see Contraindications (4)]. |
|
Antimycobacterial: |
↑bedaquiline |
Bedaquiline should only be used with lopinavir and ritonavir if the benefit of co-administration outweighs the risk. |
|
Antimycobacterial: |
↑ rifabutin and rifabutin metabolite |
Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (i.e., a maximum dose of 150 mg every other day or three times per week). Increased monitoring for adverse reactions is warranted in patients receiving the combination. Further dosage reduction of rifabutin may be necessary. |
|
Antiparasitic: |
↓ atovaquone |
Clinical significance is unknown; however, increase in atovaquone doses may be needed. |
|
Antipsychotics: pimozide |
↑ lurasidone ↑ pimozide |
Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
|
Antipsychotics: |
↑ quetiapine |
Initiation of lopinavir and ritonavir in patients taking quetiapine: |
|
Contraceptive: |
↓ ethinyl estradiol |
Because contraceptive steroid concentrations may be altered when lopinavir and ritonavir is co-administered with oral contraceptives or with the contraceptive patch, alternative methods of nonhormonal contraception are recommended. |
|
Dihydropyridine Calcium Channel Blockers: e.g. felodipine, |
↑ dihydropyridine |
Clinical monitoring of patients is recommended and a dose reduction of the dihydropyridine calcium channel blocker may be considered. |
|
Disulfiram/metronidazole |
Lopinavir and ritonavir oral solution contains alcohol, which can produce disulfiram-like reactions when co-administered with disulfiram or other drugs that produce this reaction (e.g., metronidazole). | |
|
Endothelin Receptor Antagonists: |
↑ bosentan |
Co-administration of bosentan in patients on lopinavir and ritonavir: |
|
Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine |
↑ ergot derivatives |
Contraindicated due to potential for acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [see Contraindications ( 4)]. |
|
GI Motility Agent: |
↑ cisapride |
Contraindicated due to potential for cardiac arrhythmias [see Contraindications (4)]. |
|
GnRH Receptor Antagonists: elagolix |
↑ elagolix |
Concomitant use of elagolix 200 mg twice daily and lopinavir and ritonavir for more than 1 month is not recommended due to potential risk of adverse events such as bone loss and hepatic transaminase elevations. Limit concomitant use of elagolix 150 mg once daily and lopinavir and ritonavir to 6 months. |
|
Hepatitis C direct acting antiviral: elbasvir/grazoprevir |
↑ elbasvir/grazoprevir |
Contraindicated due to increased risk of alanine transaminase (ALT) elevations [see Contraindications (4)]. |
|
Hepatitis C direct acting antivirals: simeprevir sofosbuvir/velpatasvir/voxilaprevir ombitasvir/paritaprevir/ritonavir and dasabuvir* |
↓ lopinavir ↑ sofosbuvir ↑ ombitasvir |
It is not recommended to co-administer lopinavir and ritonavirand boceprevir,
glecaprevir/pibrentasvir, simeprevir, |
|
Herbal Products: St. John's Wort (hypericum perforatum) |
↓ lopinavir |
Contraindicated due to potential for loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors [see Contraindications (4)]. |
|
Lipid-modifying agents HMG-CoA Reductase atorvastatin Microsomal triglyceride transfer protein (MTTP) Inhibitor: |
↑ lovastatin ↑ atorvastatin ↑ lomitapide |
Contraindicated due to potential for myopathy including rhabdomyolysis [see Contraindications (4)]. Use atorvastatin with caution and at the lowest necessary dose. Titrate rosuvastatin dose carefully and use the lowest necessary dose; do not exceed rosuvastatin 10 mg/day. Lomitapide is a sensitive substrate for CYP3A4 metabolism. CYP3A4 inhibitors increase the exposure of lomitapide, with strong inhibitors increasing exposure approximately 27-fold. Concomitant use of moderate or strong CYP3A4 inhibitors with lomitapide is contraindicated due to potential for hepatotoxicity [see Contraindications (4)]. |
|
Immunosuppressants: e.g. |
↑ immunosuppressants |
Therapeutic concentration monitoring is recommended for immunosuppressant agents when co-administered with lopinavir and ritonavir. |
|
Kinase Inhibitors: |
↑ fostamatinib metabolite R406 |
Monitor for toxicities of R406 such as hepatotoxicity and neutropenia. Fostamatinib dose reduction may be required. |
|
Long-acting beta-adrenoceptor Agonist: |
↑ salmeterol |
Concurrent administration of salmeterol and lopinavir and ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
|
Narcotic Analgesics: |
↓ methadone |
Dosage of methadone may need to be increased when co-administered with lopinavir and ritonavir. Careful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl is concomitantly administered with lopinavir and ritonavir. |
|
PDE5 inhibitors: |
↑ avanafil |
Sildenafil when used for the treatment of pulmonary arterial hypertension (Revatio ®) is contraindicated due to the potential for sildenafil-associated adverse events, including visual abnormalities, hypotension, prolonged erection, and syncope [see Contraindications (4)]. Do not use lopinavir and ritonavir with avanafil because a safe and effective avanafil dosage regimen has not been established. Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients receiving lopinavir and ritonavir. Coadministration of lopinavir and ritonavir with these drugs may result in an increase in PDE5 inhibitor associated adverse reactions including hypotension, syncope, visual changes and prolonged erection. Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH):
Use with increased monitoring for adverse events. |
|
Sedative/Hypnotics: |
↑ triazolam |
Contraindicated due to potential for prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
|
Sedative/Hypnotics: |
↑ midazolam |
If lopinavir and ritonavir is co-administered with parenteral midazolam, close clinical monitoring for respiratory depression and/or prolonged sedation should be exercised and dosage adjustment should be considered. |
|
Systemic/Inhaled/Nasal/ Ophthalmic Corticosteroids: e.g., betamethasone |
↓ lopinavir |
Coadministration with oral dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to lopinavir. Consider alternative corticosteroids. Coadministration with corticosteroids whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing’s syndrome and adrenal suppression. Alternative corticosteroids including beclomethasone and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use. |
refers to interaction with apalutamide. |
7.4 Drugs with No Observed or Predicted Interactions with Lopinavir and
Ritonavir
Drug interaction or clinical studies reveal no clinically significant
interaction between lopinavir and ritonavir and desipramine (CYP2D6 probe),
etravirine, pitavastatin, pravastatin, stavudine, lamivudine, omeprazole,
raltegravir, ranitidine, or rilpivirine.
Based on known metabolic profiles, clinically significant drug interactions
are not expected between lopinavir and ritonavir and dapsone,
trimethoprim/sulfamethoxazole, azithromycin, erythromycin, or fluconazole.
Co-administration of lopinavir and ritonavir can alter the plasma concentrations of other drugs and other drugs may alter the plasma concentrations of lopinavir. The potential for drug-drug interactions must be considered prior to and during therapy. (4,5.1,7,12.3)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Contraindications (4) 12/2019
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
• Lopinavir and Ritonavir Tablets USP, 200 mg lopinavir USP/50 mg ritonavir
USP
Yellow, film coated, ovaloid tablets debossed with ‘H’ on one side and ‘70’ on
other side.
• Lopinavir and Ritonavir Tablets USP, 100 mg lopinavir USP/25 mg ritonavir
USP
Yellow, capsule shaped, biconvex film coated tablets, debossed with ‘H’ on one
side and ‘L7’ on other side.
• Film coated Tablets: 200 mg lopinavir, USP and 50 mg ritonavir, USP (3)
• Film coated Tablets: 100 mg lopinavir, USP and 25 mg ritonavir, USP (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in
women exposed to lopinavir and ritonavir during pregnancy. Physicians are
encouraged to register patients by calling the Antiretroviral Pregnancy
Registry at 1-800-258-4263.
Risk Summary
Available data from the Antiretroviral Pregnancy Registry show no difference
in the risk of overall major birth defects compared to the background rate for
major birth defects of 2.7% in the U.S. reference population of the
Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data). The
estimated background rate of miscarriage in clinically recognized pregnancies
in the U.S. general population is 15 to 20%. The background risk for major
birth defects and miscarriage for the indicated population is unknown.
Methodological limitations of the APR include the use of MACDP as the external
comparator group. The MACDP population is not disease-specific, evaluates
women and infants from a limited geographic area, and does not include
outcomes for births that occurred at <20 weeks gestation (see Data). No
treatment-related malformations were observed when lopinavir in combination
with ritonavir was administered to pregnant rats or rabbits; however embryonic
and fetal developmental toxicities occurred in rats administered maternally
toxic doses.
Clinical Considerations
Dose Adjustments During Pregnancy and the Postpartum Period
Administer 400/100 mg of lopinavir and ritonavir twice daily in pregnant
patients with no documented lopinavir-associated resistance substitutions [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)]. There are
insufficient data to recommend lopinavir and ritonavir dosing for pregnant
patients with any documented lopinavir-associated resistance substitutions. No
dose adjustment of lopinavir and ritonavir is required for patients during the
postpartum period.
Once daily lopinavir and ritonavir dosing is not recommended in pregnancy.
Avoid use of lopinavir and ritonavir oral solution during pregnancy due to the
alcohol content. Lopinavir and ritonavir oral solution contains the excipients
alcohol and propylene glycol.
Data
Human Data
Lopinavir and ritonavir was evaluated in 12 HIV-infected pregnant women in an
open-label pharmacokinetic trial [see Clinical Pharmacology (12.3)]. No new
trends in the safety profile were identified in pregnant women dosed with
lopinavir and ritonavir compared to the safety described in non-pregnant
adults, based on the review of these limited data.
Antiretroviral Pregnancy Registry Data: Based on prospective reports from the
Antiretroviral Pregnancy Registry (APR) of over 3,000 exposures to lopinavir
containing regimens (including over 1,000 exposed in the first trimester),
there was no difference between lopinavir and overall birth defects compared
with the background birth defect rate of 2.7% in the U.S. reference population
of the Metropolitan Atlanta Congenital Defects Program. The prevalence of
birth defects in live births was 2.1% (95% CI:1.4%-3.0%) following first-
trimester exposure to lopinavir-containing regimens and 3.0% (95% CI:
2.4%-3.8%) following second and third trimester exposure to lopinavir-
containing regimens. Based on prospective reports from the APR of over 5,000
exposures to ritonavir containing regimens (including over 2,000 exposures in
the first trimester) there was no difference between ritonavir and overall
birth defects compared with the U.S. background rate (MACDP). The prevalence
of birth defects in live births was 2.2% (95% CI: 1.7%-2.8%) following first-
trimester exposure to ritonavir-containing regimens and 2.9% (95% CI:
2.4%-3.6%) following second and third trimester exposure to ritonavir-
containing regimens. For both lopinavir and ritonavir, sufficient numbers of
first trimester exposures have been monitored to detect at least a 1.5 fold
increase in risk of overall birth defects and a 2 fold increase in risk of
birth defects in the cardiovascular and genitourinary systems.
Animal Data
Embryonic and fetal developmental toxicities (early resorption, decreased
fetal viability, decreased fetal body weight, increased incidence of skeletal
variations and skeletal ossification delays) occurred in rats administered
lopinavir in combination with ritonavir (on gestation days 6 to 17) at a
maternally toxic dosage. Based on AUC measurements, the drug exposures in rats
at the toxic doses were approximately 0.7 times (for lopinavir) and 1.8 times
(for ritonavir) the exposures in humans at the recommended therapeutic dose
(400/100 mg twice daily). In a pre- and post-natal study in rats, a
developmental toxicity (a decrease in survival in pups between birth and
postnatal Day 21) occurred.
No embryonic and fetal developmental toxicities were observed in rabbits
administered lopinavir in combination with ritonavir (on gestation days 6 to
18) at a maternally toxic dosage. Based on AUC measurements, the drug
exposures in rabbits at the toxic doses were approximately 0.6 times (for
lopinavir) and similar to (for ritonavir) the exposures in humans at the
recommended therapeutic dose (400/100 mg twice daily).
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Because of the potential for: 1) HIV transmission (in HIV-negative infants), 2) developing viral resistance (in HIV-positive infants), and 3) adverse reactions in the breastfed infant, instruct mothers not to breastfeed if they are receiving lopinavir and ritonavir.
8.3 Females and Males of Reproductive Potential
Contraception
Use of lopinavir and ritonavir may reduce the efficacy of combined hormonal contraceptives. Advise patients using combined hormonal contraceptives to use an effective alternative contraceptive method or an additional barrier method of contraception [see Drug Interactions (7.3)].
8.4 Pediatric Use
The safety, efficacy, and pharmacokinetic profiles of lopinavir and ritonavir
in pediatric patients below the age of 14 days have not been established.
Lopinavir and ritonavir should not be administered once daily in pediatric
patients.
An open-label, multi-center, dose-finding trial was performed to evaluate the
pharmacokinetic profile, tolerability, safety and efficacy of lopinavir and
ritonavir oral solution containing lopinavir 80 mg/mL and ritonavir 20 mg/mL
at a dose of 300/75 mg/m 2 twice daily plus two NRTIs in HIV-infected infants
≥14 days and < 6 months of age. Results revealed that infants younger than 6
months of age generally had lower lopinavir AUC 12 than older children (6
months to 12 years of age), however, despite the lower lopinavir drug exposure
observed, antiviral activity was demonstrated as reflected in the proportion
of subjects who achieved HIV-1 RNA <400 copies/mL at Week 24 [see Adverse Reactions (6.2),Clinical Pharmacology (12.3),Clinical Studies (14.4)].
Safety and efficacy in pediatric patients > 6 months of age was demonstrated
in a clinical trial in 100 patients. The clinical trial was an open-label,
multicenter trial evaluating the pharmacokinetic profile, tolerability,
safety, and efficacy of lopinavir and ritonavir oral solution containing
lopinavir 80 mg/mL and ritonavir 20 mg/mL in 100 antiretroviral naïve and
experienced pediatric patients ages 6 months to 12 years. Dose selection for
patients 6 months to 12 years of age was based on the following results. The
230/57.5 mg/m 2 oral solution twice daily regimen without nevirapine and the
300/75 mg/m 2 oral solution twice daily regimen with nevirapine provided
lopinavir plasma concentrations similar to those obtained in adult patients
receiving the 400/100 mg twice daily regimen (without nevirapine) [see Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
A prospective multicenter, open-label trial evaluated the pharmacokinetic
profile, tolerability, safety and efficacy of high-dose lopinavir and
ritonavir with or without concurrent NNRTI therapy (Group 1: 400/100 mg/m 2
twice daily + ≥ 2 NRTIs; Group 2: 480/120 mg/m2 twice daily + ≥ 1 NRTI + 1
NNRTI) in 26 children and adolescents ≥ 2 years to < 18 years of age who had
failed prior therapy. Patients also had saquinavir mesylate added to their
regimen. This strategy was intended to assess whether higher than approved
doses of lopinavir and ritonavir could overcome protease inhibitor cross-
resistance. High doses of lopinavir and ritonavir exhibited a safety profile
similar to those observed in previous trials; changes in HIV-1 RNA were less
than anticipated; three patients had HIV-1 RNA <400 copies/mL at Week 48. CD4+
cell count increases were noted in the eight patients who remained on
treatment for 48 weeks [see Adverse Reactions (6.2), Clinical Pharmacology (12.3)].
A prospective multicenter, randomized, open-label study evaluated the efficacy
and safety of twice-daily versus once-daily dosing of lopinavir and ritonavir
tablets dosed by weight as part of combination antiretroviral therapy (cART)
in virologically suppressed HIV-1 infected children (n=173). Children were
eligible when they were aged < 18 years, ≥ 15 kg in weight, receiving cART
that included lopinavir and ritonavir tablets, HIV-1 ribonucleic acid (RNA) <
50 copies/mL for at least 24 weeks and able to swallow tablets. At week 24,
efficacy (defined as the proportion of subjects with plasma HIV-1 RNA less
than 50 copies per mL) was significantly higher in subjects receiving twice
daily dosing compared to subjects receiving once daily dosing. The safety
profile was similar between the two treatment arms although there was a
greater incidence of diarrhea in the once daily treated subjects.
8.5 Geriatric Use
Clinical studies of lopinavir and ritonavir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, appropriate caution should be exercised in the administration and monitoring of lopinavir and ritonavir in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Lopinavir and ritonavir is principally metabolized by the liver; therefore, caution should be exercised when administering this drug to patients with hepatic impairment, because lopinavir concentrations may be increased [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
Lactation: Breastfeeding not recommended. ( 8.2)
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdoses with lopinavir and ritonavir oral solution have been reported. One
of these reports described fatal cardiogenic shock in a 2.1 kg infant who
received a single dose of 6.5 mL of lopinavir and ritonavir oral solution (520
mg lopinavir, approximately 10-fold above the recommended lopinavir dose) nine
days prior. The following events have been reported in association with
unintended overdoses in preterm neonates: complete AV block, cardiomyopathy,
lactic acidosis, and acute renal failure [see Warnings and Precautions (5.2)].
Healthcare professionals should be aware that lopinavir and ritonavir oral
solution is highly concentrated and therefore, should pay special attention to
accurate calculation of the dose of lopinavir and ritonavir, transcription of
the medication order, dispensing information and dosing instructions to
minimize the risk for medication errors and overdose. This is especially
important for infants and young children.
Lopinavir and ritonavir oral solution contains alcohol and propylene glycol.
Ingestion of the product over the recommended dose by an infant or a young
child could result in significant toxicity and could potentially be lethal.
Human experience of acute overdosage with lopinavir and ritonavir is limited.
Treatment of overdose with lopinavir and ritonavir should consist of general
supportive measures including monitoring of vital signs and observation of the
clinical status of the patient. There is no specific antidote for overdose
with lopinavir and ritonavir. If indicated, elimination of unabsorbed drug
should be achieved by gastric lavage. Administration of activated charcoal may
also be used to aid in removal of unabsorbed drug. Since lopinavir is highly
protein bound, dialysis is unlikely to be beneficial in significant removal of
the drug. However, dialysis can remove both alcohol and propylene glycol in
the case of overdose with lopinavir and ritonavir oral solution.
SPL UNCLASSIFIED SECTION
Medication Guide
Lopinavir (loe pin' a veer) and
** Ritonavir (ri toe' na veer)**
** Tablets USP**
What is the most important information I should know about lopinavir and ritonavir tablets?
Lopinavir and ritonavir tablets may cause serious side effects, including:
•Interactions with other medicines. It is important to know the medicines
that should not be taken with lopinavir and ritonavir tablets. For more
information, see "Who should not take lopinavir and ritonavir tablets?”
•Side Effects in babies taking lopinavir and ritonavir oral solution.
Lopinavir and ritonavir oral solution contains alcohol and propylene glycol.
Call your healthcare provider right away if your baby appears too sleepy or
their breathing changes.
•**Inflammation of your pancreas (pancreatitis).**Lopinavir and ritonavir
tablets can cause pancreatitis which may be serious and may lead to death.
People who have high levels of a certain fat (triglycerides) have a risk for
developing pancreatitis. If you have advanced HIV-1 disease, you may have an
increased risk of high triglyceride levels in your blood, and pancreatitis. If
you have a history of pancreatitis, you may have an increased risk of it
coming back again during treatment with lopinavir and ritonavir tablets. Tell
your healthcare provider if you have any signs or symptoms of pancreatitis
including:
o nausea o vomiting o stomach-area (abdominal) pain
•Liver problems. Liver problems, including death, can happen in people
who take lopinavir and ritonavir tablets. Your healthcare provider should do
blood tests before and during your treatment with lopinavir and ritonavir
tablets to check your liver function. If you have Hepatitis B or Hepatitis C,
or other liver problems, you may have an increased risk for developing new or
worsening of liver problems during treatment with lopinavir and ritonavir
tablets. Tell your healthcare provider right away if you have any signs and
symptoms of liver problems including:
o loss of appetite o pale colored stools
o yellow skin and whites of eyes (jaundice) o itchy skin
o dark-colored urine o stomach area (abdominal) pain
•Changes in your heart rhythm and the electrical activity of your heart.
These changes may be seen on an EKG (electrocardiogram) and can lead to
serious heart problems. Your risk for these problems may be higher if you:
o have a history of abnormal heart rhythm or certain types of heart problems.
o take other medicines that can affect your heart rhythm during treatment with
lopinavir and ritonavir tablets.
Tell your healthcare provider right away if you have any of these symptoms:
o dizziness o fainting
o lightheadedness o sensation of abnormal heartbeats
See**“What are the possible side effects of lopinavir and ritonavir tablets?” **for more information about serious side effects.
What are lopinavir and ritonavir tablets?
Lopinavir and ritonavir tablets are a prescription medicine that is used with other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection in adults and children 14 days of age and older.
HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
It is not known if lopinavir and ritonavir tablets are safe and effective in children under 14 days old.
Who should not take lopinavir and ritonavir tablets?
Do not take lopinavir and ritonavir tablets if you:
• are allergic to lopinavir, ritonavir, or any of the ingredients in lopinavir
and ritonavir tablets. See the end of this Medication Guide for a complete
list of ingredients in lopinavir and ritonavir tablets.
• if you take any of the following medicines:
o alfuzosin
o apalutamide
o ranolazine
o dronedarone
o colchicine, if you have kidney or liver problems
o rifampin
o lurasidone
o pimozide
o ergot containing medicines including:
•dihydroergotamine mesylate
•ergotamine tartrate
•methylergonovine
o cisapride
o elbasvir/grazoprevir
o lovastatin
o simvastatin
o lomitapide
o sildenafil (Revatio®), when used for the treatment of pulmonary arterial
hypertension
o triazolam
o midazolam when taken by mouth
o St. John’s Wort (Hypericum perforatum®)
Serious problems can happen if you or your child takes any of the medicines listed above with lopinavir and ritonavir tablets.
Before taking lopinavir and ritonavir tablets, tell your healthcare provider about all of your medical conditions, including if you:
• have ever had a serious skin rash or an allergic reaction to medicines that
contain lopinavir or ritonavir.
• have or had pancreas problems.
• have liver problems, including Hepatitis B or Hepatitis C.
• have any heart problems, including if you have a condition called Congenital
Long QT Syndrome.
• have low potassium in your blood.
• have diabetes.
• have high cholesterol in your blood.
• have hemophilia. Lopinavir and ritonavir tablets may cause increased
bleeding.
• are pregnant or plan to become pregnant. It is not known if lopinavir and
ritonavir tablets will harm your unborn baby.
o Lopinavir and ritonavir oral solution contains alcohol and propylene glycol.
You should not take lopinavir and ritonavir oral solution during pregnancy
because there is no safe level of alcohol exposure during pregnancy. Tell your
healthcare provider if you become pregnant during treatment with lopinavir and
ritonavir oral solution.
o Lopinavir and ritonavir tablets may reduce how well hormonal birth control
works. Females who may become pregnant should use another effective form of
birth control or an additional barrier method of birth control during
treatment with lopinavir and ritonavir tablets.
o Pregnancy Registry: There is a pregnancy registry for women who take
antiretroviral medicines during pregnancy. The purpose of the pregnancy
registry is to collect information about the health of you and your baby. Talk
to your healthcare provider about how you can take part in this registry.
• are breastfeeding or plan to breastfeed.** Do not breastfeed if you take
lopinavir and ritonavir tablets**.
o You should not breastfeed if you have HIV-1 because of the risk of passing
HIV-1 to your baby.
o Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Many medicines interact with lopinavir and ritonavir tablets.
Keep a list of your medicines to show your healthcare provider and
pharmacist.
You can ask your healthcare provider or pharmacist for a list of medicines
that interact with lopinavir and ritonavir tablets.
Do not start taking a new medicine without telling your healthcare
provider. Your healthcare provider can tell you if it is safe to take
lopinavir and ritonavir tablets with other medicines. Your healthcare provider
may need to change the dose of other medicines during treatment with lopinavir
and ritonavir tablets.
How should I take lopinavir and ritonavir tablets?
• Take lopinavir and ritonavir tablets every day exactly as prescribed by your
healthcare provider.
• Stay under the care of your healthcare provider during treatment with
lopinavir and ritonavir tablets.
• It is important to set up a dosing schedule and follow it every day.
• Do not change your treatment or stop treatment without first talking with
your healthcare provider.
• Swallow lopinavir and ritonavir tablets whole. Do not chew, break, or crush
lopinavir and ritonavir tablets.
• Lopinavir and ritonavir tablets can be taken with or without food.
• If you are taking both didanosine and lopinavir and ritonavir tablets:
o Didanosine can be taken at the same time as lopinavir and ritonavir tablets,
without food.
o Take didanosine either 1 hour before or 2 hours after taking lopinavir and
ritonavir oral solution.
• If you are pregnant:
o Youshould nottake lopinavir and ritonavir tablets on a 1 time each day
dose schedule.
• If your child is prescribed lopinavir and ritonavir:
o Tell your healthcare provider if your child’s weight changes.
• Lopinavir and ritonavirshould notbe given to children on a 1 time each
day dose schedule. When giving lopinavir and ritonavir to your child, give
lopinavir and ritonavir exactly as prescribed.
o Use the dosing cup (supplied) or an oral syringe with mL (milliliter)
markings to give the prescribed dose of lopinavir and ritonavir oral solution
to your child. Your pharmacist should provide an oral syringe to you.
o Lopinavir and ritonavir oral solution contains propylene glycol and a large
amount of alcohol. Lopinavir and ritonavir oral solutionshould notbe
given to babies younger than 14 days of age unless your healthcare provider
thinks it is right for your baby.
• You may have a greater chance of getting diarrhea if you take lopinavir and
ritonavir tablets 1 time each day than if you take it 2 times each day.
•Do notmiss a dose of lopinavir and ritonavir tablets. This could make
the virus harder to treat. If you forget to take lopinavir and ritonavir
tablets, take the missed dose right away. If it is almost time for your next
dose,do nottake the missed dose. Instead, follow your regular dosing
schedule by taking your next dose at its regular time.Do nottake more
than one dose of lopinavir and ritonavir tablets at one time.
•If you or your child take more than the prescribed dose of lopinavir and
ritonavir tablets, call your healthcare provider or go to the nearest
emergency room right away.
What are the possible side effects of lopinavir and ritonavir tablets?
Lopinavir and ritonavir tablets can cause serious side effects, including:
• See**“What is the most important information I should know about lopinavir
and ritonavir tablets?”**
•**Diabetes and high blood sugar (hyperglycemia).**You may develop new or
worsening diabetes or high blood sugar during treatment with lopinavir and
ritonavir tablets. Tell your healthcare provider if you get any of the
following signs or symptoms:
o urinate more often than usual ◦ unusual weight loss
o increased hunger or thirst ◦ increase in your blood sugar levels
Your healthcare provider may need to start you on medicine to treat high blood sugar or change your diabetes medicines.
•Changes in your immune system (Immune Reconstitution Syndrome) can
happen when you start taking HIV-1 medicines. Your immune system may get
stronger and begin to fight infections that have been hidden in your body for
a long time. Call your healthcare provider right away if you start having new
symptoms after starting your HIV-1 medicine.
•Increases in certain fat (triglycerides and cholesterol) levels in your
blood. Large increases of triglycerides and cholesterol can be seen in blood
test results of some people who take lopinavir and ritonavir tablets. Your
healthcare provider should do blood tests to check your cholesterol and
triglyceride levels before you start taking lopinavir and ritonavir tablets
and during your treatment.
•Changes in body fatcan happen in some people who take antiretroviral
therapy. These changes may include increased amount of fat in the upper back
and neck ("buffalo hump"), breast, and around the middle of your body (trunk).
Loss of fat from the legs, arms and face may also happen. The exact cause and
long-term health effects of these conditions are not known at this time.
•Increased bleeding in people with hemophilia. Some people with
hemophilia have increased bleeding with lopinavir and ritonavir tablets or
similar medicines.
•Skin rash, which can be severe, can happen in people who take lopinavir
and ritonavir tablets. Tell your healthcare provider if you have a history of
skin rash with other medicine used to treat your HIV-1 infection or if you get
any skin rash during treatment with lopinavir and ritonavir tablets.
•Kidney stones
Common side effects of lopinavir and ritonavir tablets include:
• diarrhea • vomiting
• nausea • increased fats in blood (triglycerides or cholesterol)
These are not all of the possible side effects of lopinavir and ritonavir
tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side
effects to FDA at 1-800-FDA-1088.
How should I store lopinavir and ritonavir tablets?
• Store lopinavir and ritonavir tablets at 68° to 77°F (20° to 25°C).
• Store lopinavir and ritonavir tablets in the original container.
• Do not keep lopinavir and ritonavir tablets out of the container it comes in
for longer than 2 weeks, especially in areas where there is a lot of humidity.
• Keep the container closed tightly.
• Throw away any medicine that is out of date or that you no longer need.
Keep lopinavir and ritonavir tablets and all medicines out of the reach of children.
General information about the safe and effective use of lopinavir and ritonavir tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lopinavir and ritonavir tablets for a condition for which it was not prescribed. Do not give lopinavir and ritonavir tablets to other people, even if they have the same condition you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about lopinavir and ritonavir tablets that is written for health professionals.
What are the ingredients in lopinavir and ritonavir tablets?
Active ingredients: lopinavir USP and ritonavir USP
Inactive ingredients:
Lopinavir and Ritonavir Tablets USP, 200 mg/50 mg: colloidal silicon
dioxide, copovidone, sodium stearyl fumarate, sorbitan monolaurate and opadry
yellow which contains colloidal anhydrous silica, hypromellose, hydroxypropyl
cellulose, iron oxide yellow, polyethylene glycol, polysorbate 80, talc and
titanium dioxide.
Lopinavir and Ritonavir Tablets USP, 100 mg/25 mg: colloidal silicon
dioxide, copovidone, sodium stearyl fumarate, sorbitan monolaurate and opadry
yellow which contains colloidal anhydrous silica, hypromellose, hydroxypropyl
cellulose, iron oxide yellow, polyethylene glycol, polysorbate 80, talc and
titanium dioxide.
For more information, call 1-866-495-1995.
All brands listed are trademarks of their respective owners and are not trademarks of Hetero Labs Limited. The makers of these brands are not affiliated with and do not endorse Hetero Labs Limited or its products.
Medication Guide available at http://camberpharma.com/medication-guides

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:HETERO****TM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055,
India
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 02/2021
DESCRIPTION SECTION
11 DESCRIPTION
Lopinavir and Ritonavir Tablets, USP is a co-formulation of lopinavir USP and
ritonavir USP. Lopinavir USP is an inhibitor of the HIV-1 protease. As co-
formulated in lopinavir and ritonavir, ritonavir USP inhibits the
CYP3A-mediated metabolism of lopinavir USP, thereby providing increased plasma
levels of lopinavir USP.
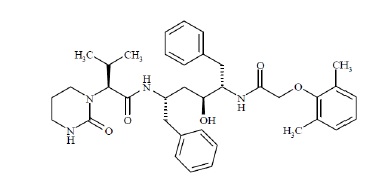
Lopinavir USP is chemically designated as [1 S-[1 R*, ( R*),3 R*,4 R*]]-
N-[4-[[(2,6-dimethylphenoxy) acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-
alpha-(1-methylethyl)-2-oxo-1(2 H)-pyrimidineacetamide. Its molecular formula
is C 37H 48N 4O 5, and its molecular weight is 628.80. Lopinavir USP is a
white to off-white powder. It is practically insoluble in water, freely
soluble in methanol, ethanol and in isopropyl alcohol. Lopinavir USP has the
following structural formula:
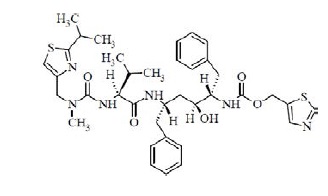
Ritonavir USP is chemically designated as 2,4,7,12-tetraazatridecan-13-oic acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C 37H 48N 6O 5S 2, and its molecular weight is 720.94. Ritonavir USP is a white to off-white powder. It is freely soluble in methanol, methylene chloride, sparingly soluble in acetonitrile and practically insoluble in water. Ritonavir USP has the following structural formula:
Lopinavir and ritonavir film coated tablets USP are available for oral
administration in two strengths:
• Yellow tablets containing 200 mg of lopinavir USP and 50 mg of ritonavir USP
• Yellow tablets containing 100 mg of lopinavir USP and 25 mg of ritonavir USP
The yellow, 200 mg lopinavir/50 mg ritonavir, tablets contain the following
inactive ingredients: colloidal silicon dioxide, copovidone, sodium stearyl
fumarate, sorbitan monolaurate and opadry yellow which contains colloidal
anhydrous silica, hypromellose, hydroxypropyl cellulose, iron oxide yellow,
polyethylene glycol, polysorbate 80, talc and titanium dioxide.
The yellow, 100 mg lopinavir/25 mg ritonavir, tablets contain the following
inactive ingredients: colloidal silicon dioxide, copovidone, sodium stearyl
fumarate, sorbitan monolaurate and opadry yellow which contains colloidal
anhydrous silica, hypromellose, hydroxypropyl cellulose, iron oxide yellow,
polyethylene glycol, polysorbate 80, talc and titanium dioxide.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lopinavir and ritonavir is a fixed-dose combination of HIV-1 antiviral drugs lopinavir [see Microbiology (12.4)] and ritonavir. As co-formulated in lopinavir and ritonavir, ritonavir inhibits the CYP3A-mediated metabolism of lopinavir, thereby providing increased plasma levels of lopinavir.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of lopinavir and ritonavir on QTcF interval was evaluated in a placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 39 healthy adults. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction were 5.3 (8.1) and 15.2 (18.0) mseconds (msec) for 400/100 mg twice daily and supratherapeutic 800/200 mg twice daily lopinavir and ritonavir, respectively. Lopinavir and ritonavir 800/200 mg twice daily resulted in a Day 3 mean C max approximately 2-fold higher than the mean C max observed with the approved once daily and twice daily lopinavir and ritonavir doses at steady state. The maximum mean (95% upper confidence bound) difference from placebo in the PR interval after baseline-correction were 24.9 (21.5, 28.3) and 31.9 (28.5, 35.3) msec for 400/100 mg twice daily and supratherapeutic 800/200 mg twice daily lopinavir and ritonavir, respectively [see Warnings and Precautions ( 5.5,5.6)].
12.3 Pharmacokinetics
The pharmacokinetic properties of lopinavir are summarized in Table 13. The steady-state pharmacokinetic parameters of lopinavir are summarized in Table 14. Under fed conditions, lopinavir concentrations were similar following administration of lopinavir and ritonavir tablets to capsules with less pharmacokinetic variability. Under fed conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of lopinavir and ritonavir capsules and oral solution.
Table 13. Pharmacokinetic Properties of Lopinavir
|
Absorption | |
|
T max (hr) a |
4.4 ± 0.8 |
|
Effect of meal (relative to fasting) Tablet |
↑ 19% b |
|
Distribution | |
|
% Bound to human plasma proteins |
|
|
V d/F a (L) |
16.9 |
|
Metabolism | |
|
Metabolism |
CYP3A |
|
Elimination | |
|
Major route of elimination |
hepatic |
|
t 1/2 (h) a |
6.9 ± 2.2 |
|
% of dose excreted in urine |
10.4 ± 2.3 |
|
% of dose excreted in feces |
82.6 ± 2.5 |
|
a. lopinavir and ritonavir tablet |
Table 14. Steady-State Pharmacokinetic Parameters of Lopinavir, Mean ± SD
|
Pharmacokinetic Parameter |
Twice Daily****a |
Once Daily****b |
|
C max (mcg/mL) |
9.8 ± 3.7 |
11.8 ± 3.7 |
|
C min (mcg/mL) |
5.5 ± 2.7 |
1.7 ± 1.6 |
|
AUC tau (mcg•h/mL) |
92.6 ± 36.7 |
154.1 ± 61.4 |
|
a. 19 HIV-1 subjects, lopinavir and ritonavir 400/100 mg twice daily |
Specific Populations
Gender, Race and Age
No gender or race related pharmacokinetic differences have been observed in adult patients. Lopinavir pharmacokinetics have not been studied in elderly patients.
Pediatric Patients
The 230/57.5 mg/m 2 twice daily regimen without nevirapine and the 300/75 mg/m 2 twice daily regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg twice daily regimen without nevirapine.
Table 15. Lopinavir Pharmacokinetic Data from Pediatric Clinical Trials, Mean ± SD
|
**Cmax (**mcg/mL) |
**Cmin(**mcg/mL) |
**AUC12 (**mcg•hr/m) |
|
Age ≥ 14 Days to < 6 Weeks Cohort (N = 9): | ||
|
5.17 ± 1.84 a |
1.40 ± 0.48 a |
43.39 ± 14.80 a |
|
Age ≥ 6 Weeks to < 6 Months Cohort (N = 18): | ||
|
9.39 ± 4.91 a |
1.95 ± 1.80 a |
74.50 ± 37.87 a |
|
Age ≥ 6 Months to ≤ 12 years Cohort (N = 24): | ||
|
8.2 ± 2.9 b |
3.4 ± 2.1 b |
72.6 ± 31.1 b |
|
10.0 ± 3.3 c |
3.6 ± 3.5 c |
85.8 ± 36.9 c |
|
a. lopinavir and ritonavir oral solution 300/75 mg/m 2 twice daily without
concomitant NNRTI therapy |
Pregnancy
The C 12h values of lopinavir were lower during the second and third trimester
by approximately 40% as compared to post-partum in 12 HIV-infected pregnant
women received lopinavir and ritonavir 400 mg/100 mg twice daily. Yet this
decrease is not considered clinically relevant in patients with no documented
lopinavir and ritonavir-associated resistance substitutions receiving 400
mg/100 mg twice daily [see Use in Specific Populations (8.1)].
Renal Impairment
Lopinavir pharmacokinetics have not been studied in patients with renal
impairment; however, since the renal clearance of lopinavir is negligible, a
decrease in total body clearance is not expected in patients with renal
impairment.
Hepatic Impairment
Multiple dosing of lopinavir and ritonavir 400/100 mg twice daily to HIV-1 and
HCV co-infected patients with mild to moderate hepatic impairment (n = 12)
resulted in a 30% increase in lopinavir AUC and 20% increase in C max compared
to HIV-1 infected subjects with normal hepatic function (n = 12).
Additionally, the plasma protein binding of lopinavir was statistically
significantly lower in both mild and moderate hepatic impairment compared to
controls (99.09 vs. 99.31%, respectively). Lopinavir and ritonavir has not
been studied in patients with severe hepatic impairment [see Warnings and Precautions (5.4)and Use in Specific Populations (8.6)].
Drug Interactions
Lopinavir and ritonavir is an inhibitor of the P450 isoform CYP3A in vitro.
Lopinavir and ritonavir does not inhibit CYP2D6, CYP2C9, CYP2C19, CYP2E1,
CYP2B6 or CYP1A2 at clinically relevant concentrations.
Lopinavir and ritonavir has been shown in vivo to induce its own metabolism
and to increase the biotransformation of some drugs metabolized by cytochrome
P450 enzymes and by glucuronidation.
The effects of co-administration of lopinavir and ritonavir on the AUC, C max
and Cmin are summarized in Table 16 (effect of other drugs on lopinavir) and
Table 17 (effect of lopinavir and ritonavir on other drugs). For information
regarding clinical recommendations, see Table 12 in Drug Interactions (7).
Table 16. Drug Interactions: Pharmacokinetic Parameters for Lopinavir in the Presence of the Co-administered Drug for Recommended Alterations in Dose or Regimen
|
Co-administered Drug |
Dose of Co-administered Drug (mg) |
Dose of Lopinavir and Ritonavir (mg) |
n |
Ratio (in combination with Co-administered drug/alone) of Lopinavir
Pharmacokinetic Parameters (90% CI); | ||
|---|---|---|---|---|---|---|
|
C****max |
AUC |
C****min | ||||
|
Efavirenz 1 |
600 at bedtime |
400/100 capsule |
11, |
0.97 |
0.81 |
0.61 |
|
600 at bedtime |
500/125 tablet |
19 |
1.12 |
1.06 |
0.90 | |
|
600 at bedtime |
600/150 tablet |
23 |
1.36 |
1.36 |
1.32 | |
|
Etravirine |
200 twice daily |
400/100 mg twice day (tablets) |
16 |
0.89 |
0.87 |
0.80 |
|
Fosamprenavir 2 |
700 twice daily plus |
400/100 capsule |
18 |
1.30 |
1.37 |
1.52 |
|
Ketoconazole |
200 single dose |
400/100 capsule |
12 |
0.89 |
0.87 |
0.75 |
|
Nelfinavir |
1000 twice daily |
400/100 capsule |
13 |
0.79 |
0.73 |
0.62 |
|
Nevirapine |
200 twice daily |
400/100 capsule |
22, |
0.81 |
0.73 |
0.49 |
|
7 mg/kg or 4 mg/kg |
(> 1 yr) |
12, |
0.86 |
0.78 |
0.45 | |
|
Ombitasvir/ paritaprevir/ ritonavir+ |
25/150/100 + dasabuvir 400 |
400/100 tablet twice daily |
6 |
0.87 |
0.94 |
1.15 |
|
Omeprazole |
40 once daily, 5 d |
400/100 tablet |
12 |
1.08 |
1.07 |
1.03 |
|
40 once daily, 5 d |
800/200 tablet |
12 |
0.94 |
0.92 |
0.71 | |
|
Pravastatin |
20 once daily, 4 d |
400/100 capsule |
12 |
0.98 |
0.95 |
0.88 |
|
Ranitidine |
150 single dose |
400/100 tablet |
12 |
0.99 |
0.97 |
0.90 |
|
150 single dose |
800/200 tablet |
10 |
0.97 |
0.95 |
0.82 | |
|
Rifabutin |
150 once daily |
400/100 capsule twice daily |
14 |
1.08 (0.97, 1.19) |
1.17 (1.04, 1.31) |
1.20 (0.96, 1.65) |
|
Rifampin |
600 once daily |
400/100 capsule |
22 |
0.45 |
0.25 |
0.01 |
|
600 once daily |
800/200 capsule |
10 |
1.02 |
0.84 |
0.43 | |
|
600 once daily |
400/400 capsule |
9 |
0.93 |
0.98 |
1.03 | |
|
Rilpivirine |
150 once daily |
400/100 twice daily (capsules) |
15 |
0.96 |
0.99 |
0.89 |
|
Ritonavir |
100 twice daily |
400/100 capsule |
8, |
1.28 |
1.46 |
2.16 |
|
Tipranavir/ |
500/200 mg twice |
400/100 capsule |
21 |
0.53 |
0.45 |
0.30 |
|
1 Reference for comparison is lopinavir/ritonavir 400/100 mg twice daily
without efavirenz. |
Table 17. Drug Interactions: Pharmacokinetic Parameters for Co- administered Drug in the Presence of Lopinavir and Ritonavir for Recommended Alterations in Dose or Regimen
|
Co-administered Drug |
Dose of |
Dose of Lopinavir and Ritonavir |
n |
Ratio (in combination with lopinavir and ritonavir/alone) of Co-administered
Drug Pharmacokinetic Parameters (90% CI); | ||
|
C****max |
AUC |
C****min | ||||
|
Bedaquiline 1 |
400 single dose |
400/100 twice daily |
N/A |
N/A |
1.22 |
N/A |
|
Efavirenz |
600 at bedtime |
400/100 |
11, |
0.91 |
0.84 |
0.84 |
|
Elbasvir/ |
50 once daily |
400/100 twice daily |
10 |
2.87 (2.29, 3.58) |
3.71 (3.05, 4.53) |
4.58 (3.72, 5.64) |
|
200 once daily |
13 |
7.31 (5.65, 9.45) |
12.86 (10.25, 16.13) |
21.70 (12.99, 36.25) | ||
|
Ethinyl Estradiol |
35 mcg once daily |
400/100 |
12 |
0.59 |
0.58 |
0.42 |
|
Etravirine |
200 twice daily |
400/100 tablet twice day |
16 |
0.70 (0.64-0.78) |
0.65 (0.59-0.71) |
0.55 (0.49-0.62) |
|
Fosamprenavir 1 |
700 twice daily plus ritonavir |
400/100 |
18 |
0.42 |
0.37 |
0.35 |
|
Indinavir |
600 twice daily combo |
400/100 |
13 |
0.71 |
0.91 |
3.47 |
|
Ketoconazole |
200 single dose |
400/100 capsule |
12 |
1.13 |
3.04 |
N/A |
|
Maraviroc 1 |
300 twice daily |
400/100 twice daily |
11 |
1.97 |
3.95 |
9.24 |
|
Methadone |
5 single dose |
400/100 |
11 |
0.55 |
0.47 |
N/A |
|
Nelfinavir |
1000 twice daily combo vs. 1250 twice daily alone |
400/100 capsule twice daily |
13 |
0.93 |
1.07 |
1.86 |
|
M8 metabolite |
2.36 |
3.46 |
7.49 | |||
|
Nevirapine |
200 once daily |
400/100 |
5, 6 3 |
1.05 |
1.08 |
1.15 |
|
Norethindrone |
1 once daily (Ortho |
400/100 |
12 |
0.84 |
0.83 |
0.68 |
|
Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir 1 |
25/150/100 + dasabuvir 400 |
400/100 tablet twice daily |
6 |
1.14 |
1.17 |
1.24 |
|
2.04 |
2.17 |
2.36 | ||||
|
1.55 |
2.05 |
5.25 | ||||
|
0.99 (0.75, 1.31) |
0.93 |
0.68 | ||||
|
Pitavastatin 1 |
4 once daily |
400/100 tablet twice daily |
23 |
0.96 |
0.80 |
N/A |
|
Pravastatin |
20 once daily |
400/100 capsule |
12 |
1.26 |
1.33 |
N/A |
|
Rifabutin |
150 once daily, combo vs. 300 once daily alone |
400/100 |
12 |
2.12 |
3.03 |
4.90 |
|
25- O-desacetyl |
23.6 |
47.5 |
94.9 | |||
|
Rifabutin + |
3.46 |
5.73 |
9.53 | |||
|
Rilpivirine |
150 once daily |
400/100 capsules twice daily |
15 |
1.29 |
1.52 |
1.74 |
|
Rosuvastatin 2 |
20 once daily |
400/100 tablet |
15 |
4.66 |
2.08 |
1.04 |
|
Tenofovir alafenamide 1 |
10 once daily |
800/200 tablet once daily |
10 |
2.19 (1.72, 2.79) |
1.47 (1.17, 1.85) |
N/A |
|
Tenofovir disoproxil fumarate 1 |
300 once daily |
400/100 |
24 |
No Change |
1.32 |
1.51 |
|
1 Data extracted from the U.S. prescribing information of co-administered
drugs. |
12.4 Microbiology
Mechanism of Action
Lopinavir, an inhibitor of the HIV-1 protease, prevents cleavage of the viral
Gag-Pol polyprotein, resulting in the production of immature, non-infectious
viral particles.
Antiviral Activity
In the absence of human serum, the mean 50% effective concentration (EC 50)
values of lopinavir against five different HIV-1 subtype B laboratory strains
in lymphoblastic cell lines ranged from 10 to 27 nM (0.006 to 0.017 mcg/mL, 1
mcg/mL = 1.6 µM), and ranged from 4 to 11 nM (0.003 to 0.007 mcg/mL) against
several HIV-1 subtype B clinical isolates in peripheral blood lymphocytes (n =
6). In the presence of 50% human serum, the mean EC 50 values of lopinavir
against these five HIV-1 laboratory strains ranged from 65 to 289 nM (0.04 to
0.18 mcg/mL), representing a 7 to 11-fold attenuation. The EC 50 values of
lopinavir against three different HIV-2 strains ranged from 12 to 180 nM
(0.008 to 113 mcg/mL).
Resistance
HIV-1 isolates with reduced susceptibility to lopinavir have been selected in
cell culture. The presence of ritonavir does not appear to influence the
selection of lopinavir-resistant viruses in cell culture.
In a study of 653 antiretroviral treatment-naïve patients (Study 863), plasma
viral isolates from each patient on treatment with plasma HIV-1 RNA >400
copies/mL at Week 24, 32, 40 and/or 48 were analyzed. No specific amino acid
substitutions could be associated with resistance to lopinavir and ritonavir
in the virus from 37 evaluable lopinavir and ritonavir-treated patients. The
selection of resistance to lopinavir and ritonavir in antiretroviral
treatment-naïve pediatric patients (Study 940) appears to be consistent with
that seen in adult patients (Study 863).
Resistance to lopinavir and ritonavir has been noted to emerge in patients
treated with other protease inhibitors prior to lopinavir and ritonavir
therapy. In studies of 227 antiretroviral treatment-naïve and protease
inhibitor experienced patients, isolates from 4 of 23 patients with
quantifiable (>400 copies/mL) viral RNA following treatment with lopinavir and
ritonavir for 12 to 100 weeks displayed significantly reduced susceptibility
to lopinavir compared to the corresponding baseline viral isolates. All four
of these patients had previously received treatment with at least one protease
inhibitor and had at least 4 substitutions associated with protease inhibitor
resistance immediately prior to lopinavir and ritonavir therapy. Following
viral rebound, isolates from these patients all contained additional
substitutions, some of which are recognized to be associated with protease
inhibitor resistance.
Cross-resistance - Nonclinical Studies
Varying degrees of cross-resistance have been observed among HIV-1 protease
inhibitors. The antiviral activity in cell culture of lopinavir against
clinical isolates from patients previously treated with a single protease
inhibitor was determined (Table 18).
Table 18. Susceptibility Reduction to Lopinavir Against Isolates from
Patients Previously Treated With a Single Protease Inhibitor
|
Susceptibility reduced by >4 fold |
Susceptibility reduced to LPV |
|
Indinavir (n=16) |
5.7 fold |
|
Nelfinavir (n=13) |
<4 fold |
|
Ritonavir (n=3) |
8.32 fold |
|
Saquinavir (n=4) |
<4 fold |
Isolates from patients previously treated with two or more protease inhibitors showed greater reductions in susceptibility to lopinavir, as described in the following section.
Clinical Studies - Antiviral Activity of Lopinavir and Ritonavir in Patients
with Previous Protease Inhibitor Therapies
The clinical relevance of reduced susceptibility in cell culture to lopinavir
has been examined by assessing the virologic response to lopinavir and
ritonavir therapy in treatment-experienced patients, with respect to baseline
viral genotype in three studies and baseline viral phenotype in one study.
Virologic response to lopinavir and ritonavir has been shown to be affected by
the presence of three or more of the following amino acid substitutions in
protease at baseline: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V,
I54L/T/V, V82A/C/F/S/T, and I84V. Table 19 shows the 48-week virologic
response (HIV-1 RNA <400 copies/mL) according to the number of the above
protease inhibitor resistance-associated substitutions at baseline in studies
888 and 765 [see Clinical Studies (14.2)and (14.3)] and study 957 (see below).
Once daily administration of lopinavir and ritonavir for adult patients with
three or more of the above substitutions is not recommended.
**Table 19. Virologic Response (HIV-1 RNA <400 copies/mL) at Week 48 by Baseline Lopinavir and Ritonavir Susceptibility and by Number of Protease Substitutions Associated with Reduced Response to Lopinavir and Ritonavir **1
|
Number of protease |
Study 888 (Single protease inhibitor-experienced2, |
Study 765 (Single protease inhibitor-experienced3, |
Study 957 (Multiple protease inhibitor-experienced4, |
|
0-2 |
76/103 (74%) |
34/45 (76%) |
19/20 (95%) |
|
3-5 |
13/26 (50%) |
8/11 (73%) |
18/26 (69%) |
|
6 or more |
0/1 (0%) |
N/A |
1/4 (25%) |
|
1 Substitutions considered in the analysis included L10F/I/R/V, K20M/N/R,
L24I, L33F, M36I, I47V, |
Virologic response to lopinavir and ritonavir therapy with respect to
phenotypic susceptibility to lopinavir at baseline was examined in Study 957.
In this study 56 NNRTI-naïve patients with HIV-1 RNA >1,000 copies/mL despite
previous therapy with at least two protease inhibitors selected from
indinavir, nelfinavir, ritonavir, and saquinavir were randomized to receive
one of two doses of lopinavir and ritonavir in combination with efavirenz and
nucleoside reverse transcriptase inhibitors (NRTIs). The EC 50 values of
lopinavir against the 56 baseline viral isolates ranged from 0.5- to 96-fold
the wild-type EC 50 value. Fifty-five percent (31/56) of these baseline
isolates displayed >4-fold reduced susceptibility to lopinavir. These 31
isolates had a median reduction in lopinavir susceptibility of 18-fold.
Response to therapy by baseline lopinavir susceptibility is shown in Table 20.
**Table 20. HIV-1 RNA Response at Week 48 by Baseline Lopinavir Susceptibility
**1
|
Lopinavir susceptibility****2 |
HIV-1 RNA <400 copies/mL |
HIV-1 RNA <50 copies/mL |
|
< 10 fold |
25/27 (93%) |
22/27 (81%) |
|
11/15 (73%) |
9/15 (60%) |
|
³ 40 fold |
2/8 (25%) |
2/8 (25%) |
|
1 Lopinavir susceptibility was determined by recombinant phenotypic technology
performed by Virologic. |
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lopinavir/ritonavir combination was evaluated for carcinogenic potential by
oral gavage administration to mice and rats for up to 104 weeks. Results
showed an increase in the incidence of benign hepatocellular adenomas and an
increase in the combined incidence of hepatocellular adenomas plus carcinoma
in both males and females in mice and males in rats at doses that produced
approximately 1.6 to 2.2 times (mice) and 0.5 times (rats) the human exposure
(based on AUC 0 to 24hr measurement) at the recommended dose of 400/100 mg
lopinavir and ritonavir twice daily. Administration of lopinavir/ritonavir did
not cause a statistically significant increase in the incidence of any other
benign or malignant neoplasm in mice or rats.
Carcinogenicity studies in mice and rats have been carried out on ritonavir.
In male mice, there was a dose dependent increase in the incidence of both
adenomas and combined adenomas and carcinomas in the liver. Based on AUC
measurements, the exposure at the high dose was approximately 4-fold for males
that of the exposure in humans with the recommended therapeutic dose (400/100
mg lopinavir and ritonavir twice daily). There were no carcinogenic effects
seen in females at the dosages tested. The exposure at the high dose was
approximately 9-fold for the females that of the exposure in humans. There
were no carcinogenic effects in rats. In this study, the exposure at the high
dose was approximately 0.7-fold that of the exposure in humans with the
400/100 mg lopinavir and ritonavir twice daily regimen. Based on the exposures
achieved in the animal studies, the significance of the observed effects is
not known.
Mutagenesis
Neither lopinavir nor ritonavir was found to be mutagenic or clastogenic in a
battery of in vitro and in vivo assays including the Ames bacterial reverse
mutation assay using S. typhimurium and E. coli, the mouse lymphoma assay, the
mouse micronucleus test and chromosomal aberration assays in human
lymphocytes.
Impairment of Fertility
Lopinavir in combination with ritonavir at a 2:1 ratio produced no effects on
fertility in male and female rats at levels of 10/5, 30/15 or 100/50
mg/kg/day. Based on AUC measurements, the exposures in rats at the high doses
were approximately 0.7-fold for lopinavir and 1.8-fold for ritonavir of the
exposures in humans at the recommended therapeutic dose (400/100 mg twice
daily).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adult Patients without Prior Antiretroviral Therapy
Study 863: Lopinavir and Ritonavir Capsules twice daily + stavudine +
lamivudine compared to nelfinavir three times daily + stavudine + lamivudine
Study 863 was a randomized, double-blind, multicenter trial comparing
treatment with lopinavir and ritonavir capsules (400/100 mg twice daily) plus
stavudine and lamivudine versus nelfinavir (750 mg three times daily) plus
stavudine and lamivudine in 653 antiretroviral treatment naïve patients.
Patients had a mean age of 38 years (range: 19 to 84), 57% were Caucasian, and
80% were male. Mean baseline CD4+ cell count was 259 cells/mm3 (range: 2 to
949 cells/mm 3) and mean baseline plasma HIV-1 RNA was 4.9 log 10 copies/mL
(range: 2.6 to 6.8 log 10 copies/mL).
Treatment response and outcomes of randomized treatment are presented in Table
21.
Table 21. Outcomes of Randomized Treatment Through Week 48 (Study 863)
|
Outcome |
Lopinavir and Ritonavir +d4T+3TC |
Nelfinavir+d4T+3TC |
|
Responder 1 |
75% |
62% |
|
Virologic failure 2 |
9% |
25% |
|
Death |
2% |
1% |
|
Discontinued due to adverse events |
4% |
4% |
|
Discontinued for other reasons 3 |
10% |
8% |
|
1 Patients achieved and maintained confirmed HIV-1 RNA < 400 copies/mL through
Week 48. |
Through 48 weeks of therapy, there was a statistically significantly higher proportion of patients in the lopinavir and ritonavir arm compared to the nelfinavir arm with HIV-1 RNA < 400 copies/mL (75% vs. 62%, respectively) and HIV-1 RNA < 50 copies/mL (67% vs. 52%, respectively). Treatment response by baseline HIV-1 RNA level subgroups is presented in Table 22.
Table 22. Proportion of Responders Through Week 48 by Baseline Viral Load (Study 863)
|
Baseline Viral Load |
Lopinavir and Ritonavir |
Nelfinavir +d4T+3TC | ||||
|
<400 copies/mL****1 |
<50 copies/mL****2 |
n |
<400 copies/mL****1 |
<50 copies/mL****2 |
n | |
|
< 30,000 |
74% |
71% |
82 |
79% |
72% |
87 |
|
³ 30,000 to < 100,000 |
81% |
73% |
79 |
67% |
54% |
79 |
|
³ 100,000 to < 250,000 |
75% |
64% |
83 |
60% |
47% |
72 |
|
³ 250,000 |
72% |
60% |
82 |
44% |
33% |
89 |
|
1 Patients achieved and maintained confirmed HIV-1 RNA < 400 copies/mL through
Week 48. |
Through 48 weeks of therapy, the mean increase from baseline in CD4+ cell
count was 207 cells/mm 3 for the lopinavir and ritonavir arm and 195 cells/mm
3 for the nelfinavir arm.
Study 730: Lopinavir and Ritonavir Tablets once daily + tenofovir DF +
emtricitabine compared to Lopinavir and Ritonavir Tablets twice daily +
tenofovir DF + emtricitabine
Study 730 was a randomized, open-label, multicenter trial comparing treatment
with lopinavir and ritonavir 800/200 mg once daily plus tenofovir DF and
emtricitabine versus lopinavir and ritonavir 400/100 mg twice daily plus
tenofovir DF and emtricitabine in 664 antiretroviral treatment-naïve patients.
Patients were randomized in a 1:1 ratio to receive either lopinavir and
ritonavir 800/200 mg once daily (n = 333) or lopinavir and ritonavir 400/100
mg twice daily (n = 331). Further stratification within each group was 1:1
(tablet vs. capsule). Patients administered the capsule were switched to the
tablet formulation at Week 8 and maintained on their randomized dosing
schedule. Patients were administered emtricitabine 200 mg once daily and
tenofovir DF 300 mg once daily. Mean age of patients enrolled was 39 years
(range: 19 to 71); 75% were Caucasian, and 78% were male. Mean baseline CD4+
cell count was 216 cells/mm 3 (range: 20 to 775 cells/mm 3) and mean baseline
plasma HIV-1 RNA was 5.0 log 10 copies/mL (range: 1.7 to 7.0 log 10
copies/mL).
Treatment response and outcomes of randomized treatment through Week 48 are
presented in Table 23.
Table 23. Outcomes of Randomized Treatment Through Week 48 (Study 730)
|
Outcome |
Lopinavir and Ritonavir |
Lopinavir and Ritonavir |
|
Responder 1 |
78% |
77% |
|
Virologic failure 2 |
10% |
8% |
|
Death |
1% |
<1% |
|
Discontinued due to adverse events |
4% |
3% |
|
Discontinued for other reasons 3 |
8% |
11% |
|
1 Patients achieved and maintained confirmed HIV-1 RNA < 50 copies/mL through
Week 48. |
Through 48 weeks of therapy, 78% in the lopinavir and ritonavir once daily arm and 77% in the lopinavir and ritonavir twice daily arm achieved and maintained HIV-1 RNA < 50 copies/mL (95% confidence interval for the difference, -5.9% to 6.8%). Mean CD4+ cell count increases at Week 48 were 186 cells/mm 3 for the lopinavir and ritonavir once daily arm and 198 cells/mm3 for the lopinavir and ritonavir twice daily arm.
14.2 Adult Patients with Prior Antiretroviral Therapy
Study 888: Lopinavir and Ritonavir Capsules twice daily + nevirapine + NRTIs
compared to investigator-selected protease inhibitor(s) + nevirapine + NRTIs
Study 888 was a randomized, open-label, multicenter trial comparing treatment
with lopinavir and ritonavir capsules (400/100 mg twice daily) plus nevirapine
and nucleoside reverse transcriptase inhibitors versus investigator-selected
protease inhibitor(s) plus nevirapine and nucleoside reverse transcriptase
inhibitors in 288 single protease inhibitor-experienced, non-nucleoside
reverse transcriptase inhibitor (NNRTI)-naïve patients. Patients had a mean
age of 40 years (range: 18 to 74), 68% were Caucasian, and 86% were male. Mean
baseline CD4+ cell count was 322 cells/mm 3 (range: 10 to 1059 cells/mm 3) and
mean baseline plasma HIV-1 RNA was 4.1 log 10 copies/mL (range: 2.6 to 6.0 log
10 copies/mL).
Treatment response and outcomes of randomized treatment through Week 48 are
presented in Table 24.
Table 24. Outcomes of Randomized Treatment Through Week 48 (Study 888)
|
Outcome |
Lopinavir and Ritonavir +nevirapine + NRTIs |
Investigator-Selected Protease Inhibitor(s) + nevirapine + NRTIs |
|
Responder 1 |
57% |
33% |
|
Virologic failure 2 |
24% |
41% |
|
Death |
1% |
2% |
|
Discontinued due to adverse events |
5% |
11% |
|
Discontinued for other reasons 3 |
14% |
13% |
|
1 Patients achieved and maintained confirmed HIV-1 RNA < 400 copies/mL through
Week 48. |
Through 48 weeks of therapy, there was a statistically significantly higher
proportion of patients in the lopinavir and ritonavir arm compared to the
investigator-selected protease inhibitor(s) arm with HIV-1 RNA < 400 copies/mL
(57% vs. 33%, respectively).
Through 48 weeks of therapy, the mean increase from baseline in CD4+ cell
count was 111 cells/mm 3 for the lopinavir and ritonavir arm and 112 cells/mm
3 for the investigator-selected protease inhibitor(s) arm.
Study 802: Lopinavir and Ritonavir Tablets 800/200 mg Once Daily Versus
400/100 mg Twice Daily when Co-administered with Nucleoside/Nucleotide Reverse
Transcriptase Inhibitors in Antiretroviral-Experienced, HIV-1 Infected
Subjects
M06-802 was a randomized open-label study comparing the safety, tolerability,
and antiviral activity of once daily and twice daily dosing of lopinavir and
ritonavir tablets in 599 subjects with detectable viral loads while receiving
their current antiviral therapy. Of the enrolled subjects, 55% on both
treatment arms had not been previously treated with a protease inhibitor and
81 to 88% had received prior NNRTIs as part of their anti-HIV treatment
regimen. Patients were randomized in a 1:1 ratio to receive either lopinavir
and ritonavir 800/200 mg once daily (n = 300) or lopinavir and ritonavir
400/100 mg twice daily (n = 299). Patients were administered at least two
nucleoside/nucleotide reverse transcriptase inhibitors selected by the
investigator. Mean age of patients enrolled was 41 years (range: 21 to 73);
51% were Caucasian, and 66% were male. Mean baseline CD4+ cell count was 254
cells/mm 3 (range: 4 to 952 cells/mm 3) and mean baseline plasma HIV-1 RNA was
4.3 log 10 copies/mL (range: 1.7 to 6.6 log 10 copies/mL).
Treatment response and outcomes of randomized treatment through Week 48 are
presented in Table 25.
Table 25. Outcomes of Randomized Treatment Through Week 48 (Study 802)
|
Outcome |
Lopinavir and Ritonavir Once Daily + NRTIs |
Lopinavir and Ritonavir |
|
Virologic Success (HIV-1 RNA <50 copies/mL) |
57% |
54% |
|
Virologic failure 1 |
22% |
24% |
|
No virologic data in Week 48 window | ||
|
Discontinued study due to adverse event or death 2 |
5% |
7% |
|
Discontinued study for other reasons 3 |
13% |
12% |
|
Missing data during window but on study |
3% |
3% |
|
1 Includes patients who discontinued prior to Week 48 for lack or loss of
efficacy and patients with HIV-1 RNA ≥ 50 copies/mL at Week 48. |
Through 48 weeks of treatment, the mean change from baseline for CD4 + cell count was 135 cells/mm 3 for the once daily group and 122 cells/mm 3 for the twice daily group.
14.3 Other Studies Supporting Approval in Adult Patients
Study 720: Lopinavir and ritonavir twice daily + stavudine + lamivudine
Study 765: Lopinavir and ritonavir twice daily + nevirapine + NRTIs
Study 720 (patients without prior antiretroviral therapy) and study 765
(patients with prior protease inhibitor therapy) were randomized, blinded,
multi-center trials evaluating treatment with lopinavir and ritonavir at up to
three dose levels (200/100 mg twice daily [720 only], 400/100 mg twice daily,
and 400/200 mg twice daily). In Study 720, all patients switched to 400/100 mg
twice daily between Weeks 48 to 72. Patients in study 720 had a mean age of 35
years, 70% were Caucasian, and 96% were male, while patients in study 765 had
a mean age of 40 years, 73% were Caucasian, and 90% were male. Mean (range)
baseline CD4+ cell counts for patients in study 720 and study 765 were 338 (3
to 918) and 372 (72 to 807) cells/mm 3, respectively. Mean (range) baseline
plasma HIV-1 RNA levels for patients in study 720 and study 765 were 4.9 (3.3
to 6.3) and 4.0 (2.9 to 5.8) log10 copies/mL, respectively.
Through 360 weeks of treatment in study 720, the proportion of patients with
HIV-1 RNA < 400 (< 50) copies/mL was 61% (59%) [n = 100]. Among patients
completing 360 weeks of treatment with CD4+ cell count measurements [n=60],
the mean (median) increase in CD4+ cell count was 501 (457) cells/mm 3.
Thirty-nine patients (39%) discontinued the study, including 13 (13%)
discontinuations due to adverse reactions and 1 (1%) death.
Through 144 weeks of treatment in study 765, the proportion of patients with
HIV-1 RNA < 400 (< 50) copies/mL was 54% (50%) [n = 70], and the corresponding
mean increase in CD4+ cell count was 212 cells/mm 3. Twenty-seven patients
(39%) discontinued the study, including 5 (7%) discontinuations secondary to
adverse reactions and 2 (3%) deaths.
14.4 Pediatric Studies
Study 1030 was an open-label, multicenter, dose-finding trial evaluating the
pharmacokinetic profile, tolerability, safety and efficacy of lopinavir and
ritonavir oral solution containing lopinavir 80 mg/mL and ritonavir 20 mg/mL
at a dose of 300/75 mg/m 2 twice daily plus 2 NRTIs in HIV-1 infected infants
≥14 days and <6 months of age.
Ten infants, ≥14 days and <6 wks of age, were enrolled at a median (range) age
of 5.7 (3.6 to 6.0) weeks and all completed 24 weeks. At entry, median (range)
HIV-1 RNA was 6.0 (4.7 to 7.2) log 10 copies/mL. Seven of 10 infants had HIV-1
RNA <400 copies/mL at Week 24. At entry, median (range) CD4+ percentage was 41
(16 to 59) with a median decrease of 1% (95% CI: -10, 18) from baseline to
week 24 in 6 infants with available data.
Twenty-one infants, between 6 weeks and 6 months of age, were enrolled at a
median (range) age of 14.7 (6.9 to 25.7) weeks and 19 of 21 infants completed
24 weeks. At entry, median (range) HIV RNA level was 5.8 (3.7 to 6.9) log 10
copies/mL. Ten of 21 infants had HIV RNA <400 copies/mL at Week 24. At entry,
the median (range) CD4+ percentage was 32 (11 to 54) with a median increase of
4% (95% CI: -1, 9) from baseline to week 24 in 19 infants with available data
[see Clinical Pharmacology (12.3) for pharmacokinetic results].
Study 940 was an open-label, multicenter trial evaluating the pharmacokinetic
profile, tolerability, safety and efficacy of lopinavir and ritonavir oral
solution containing lopinavir 80 mg/mL and ritonavir 20 mg/mL in 100
antiretroviral naïve (44%) and experienced (56%) pediatric patients. All
patients were non-nucleoside reverse transcriptase inhibitor naïve. Patients
were randomized to either 230 mg lopinavir/57.5 mg ritonavir per m 2 or 300 mg
lopinavir/75 mg ritonavir per m 2. Naïve patients also received lamivudine and
stavudine. Experienced patients received nevirapine plus up to two nucleoside
reverse transcriptase inhibitors.
Safety, efficacy and pharmacokinetic profiles of the two dose regimens were
assessed after three weeks of therapy in each patient. After analysis of these
data, all patients were continued on the 300 mg lopinavir/75 mg ritonavir per
m2 dose. Patients had a mean age of 5 years (range 6 months to 12 years) with
14% less than 2 years. Mean baseline CD4+ cell count was 838 cells/mm3 and
mean baseline plasma HIV-1 RNA was 4.7 log 10 copies/mL.
Through 48 weeks of therapy, the proportion of patients who achieved and
sustained an HIV-1 RNA < 400 copies/mL was 80% for antiretroviral naïve
patients and 71% for antiretroviral experienced patients. The mean increase
from baseline in CD4+ cell count was 404 cells/mm 3 for antiretroviral naïve
and 284 cells/mm 3 for antiretroviral experienced patients treated through 48
weeks. At 48 weeks, two patients (2%) had prematurely discontinued the study.
One antiretroviral naïve patient prematurely discontinued secondary to an
adverse reaction, while one antiretroviral experienced patient prematurely
discontinued secondary to an HIV-1 related event.
Dose selection in pediatric patients was based on the following:
• Among patients 14 days to 6 months of age receiving 300/75 mg/m 2 twice
daily without nevirapine, plasma concentrations were lower than those observed
in adults or in older children. This dose resulted in HIV-1 RNA < 400
copies/mL in 55% of patients (70% in those initiating treatment at <6 weeks of
age).
• Among patients 6 months to 12 years of age, the 230/57.5 mg/m 2 oral
solution twice daily regimen without nevirapine and the 300/75 mg/m 2 oral
solution twice daily regimen with nevirapine provided lopinavir plasma
concentrations similar to those obtained in adult patients receiving the
400/100 mg twice daily regimen (without nevirapine). These doses resulted in
treatment benefit (proportion of patients with HIV-1 RNA < 400 copies/mL)
similar to that seen in the adult clinical trials.
• Among patients 12 to 18 years of age receiving 400/100 mg/m2 or 480/120 mg/m
2 (with efavirenz) twice daily, plasma concentrations were 60 to 100% higher
than among 6 to 12 year old patients receiving 230/57.5 mg/m 2. Mean apparent
clearance was similar to that observed in adult patients receiving standard
dose and in patients 6 to 12 years of age. Although changes in HIV-1 RNA in
patients with prior treatment failure were less than anticipated, the
pharmacokinetic data supports use of similar dosing as in patients 6 to 12
years of age, not to exceed the recommended adult dose.
• For all age groups, the body surface area dosing was converted to body
weight dosing using the patient’s prescribed lopinavir dose.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Lopinavir and ritonavir film coated tablets USP are available in the following strengths and package sizes:
Lopinavir and Ritonavir Tablets USP, 200 mg/50 mg
Yellow film coated, ovaloid tablets debossed with ‘H’ on one side and ‘70’ on other side.
Bottles of 60 tablets (NDC 31722-556-60)
Bottles of 120 tablets (NDC 31722-556-12)
Blister pack of 80 (8x10) Unit dose tablets (Alu-Alu) (NDC 31722-556-31)
Blister pack of 80 (8x10) Unit dose tablets (Alu-PVC/PVdC) (NDC 31722-556-32)
Recommended Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in original container or USP equivalent tight container (250 mL or less).
For patient use: exposure of this product to high humidity outside the original container or USP equivalent tight container (250 mL or less) for longer than 2 weeks is not recommended.
Lopinavir and Ritonavir Tablets USP, 100 mg/25 mg
Yellow, capsule shaped, biconvex film coated tablets, debossed with ‘H’ on one side and ‘L7’ on other side
Bottles of 60 tablets (NDC 31722-603-60)
Bottles of 120 tablets (NDC 31722-603-12)
Recommended Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in original container or USP equivalent tight container (100 mL or less).
For patient use: exposure of this product to high humidity outside the original container or USP equivalent tight container (100 mL or less) for longer than 2 weeks is not recommended.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
General Administration Information [see Dosage and Administration ( 2)]:
• Advise patients to pay special attention to accurate administration of their
dose to minimize the risk of accidental overdose or underdose of lopinavir and
ritonavir tablets.
• Advise caregivers to inform their healthcare provider if the child’s weight
changes in order to make sure that the child’s lopinavir and ritonavir tablets
dose is adjusted as needed.
• Inform patients and caregivers that lopinavir and ritonavir tablets may be
taken with or without food but lopinavir and ritonavir oral solution should be
taken with food to enhance absorption.
• Advise patients to remain under the care of a healthcare provider while
using lopinavir and ritonavir tablets and to take lopinavir and ritonavir
tablets in combination with other antiretroviral drugs as prescribed.
• Advise patients not to alter the dose or discontinue therapy without
consulting with their healthcare provider. If a dose of lopinavir and
ritonavir tablets is missed patients should take the dose as soon as possible
and then return to their normal schedule. However, if a dose is skipped the
patient should not double the next dose.
• Inform patients that it is important to take lopinavir and ritonavir tablets
on a regular dosing schedule as directed and to avoid missing doses as that
can result in development of resistance.
• Inform patients that there may be a greater chance of developing diarrhea
with the once daily regimen as compared with the twice daily regimen.
• Inform patients that lopinavir and ritonavir tablets are not a cure for
HIV-1 infection and that they may continue to experience illnesses associated
with HIV-1 infection, including opportunistic infections.
Drug Interactions
Inform patients that lopinavir and ritonavir tablets may interact with some drugs; therefore, patients should be advised to report to their healthcare provider the use of any prescription, non-prescription medication or herbal products such as St. John’s Wort [see Contraindications (4),Warnings and Precautions (5.1) and Drug Interactions (7)].
Pancreatitis
Advise patients that pancreatitis has been observed in patients receiving lopinavir and ritonavir tablets and to alert their healthcare provider if they experience symptoms such as nausea, vomiting or abdominal pain [see Warnings and Precautions (5.3)].
Skin Rash
Inform patients that skin rash ranging in severity from mild to toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome, erythema multiforme, urticaria, and angioedema have been reported in patients receiving lopinavir and ritonavir tablets or its components lopinavir and/or ritonavir. Advise patients to contact their healthcare provider if they develop a rash while taking lopinavir and ritonavir tablets [see Adverse Reactions (6.1)].
Hepatotoxicity
Pre-existing liver disease including Hepatitis B or C can worsen with use of
lopinavir and ritonavir tablets. This can be seen as worsening of transaminase
elevations or hepatic decompensation. Advise patients that their liver
function tests will need to be monitored closely especially during the first
several months of lopinavir and ritonavir tablets treatment and that they
should notify their healthcare provider if they develop the signs and symptoms
of worsening liver disease including loss of appetite, abdominal pain,
jaundice, and itchy skin [see Warnings and Precautions (5.4)].
QT and PR Interval Prolongation
Advise patients that lopinavir and ritonavir tablets may produce changes in the electrocardiogram (e.g., PR and/or QT prolongation) and to consult their healthcare provider if they experience symptoms such as dizziness, lightheadedness, abnormal heart rhythm or loss of consciousness [see Warnings and Precautions ( 5.5, 5.6)].
Diabetes Mellitus/Hyperglycemia
Advise patients that new onset of diabetes or exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during lopinavir and ritonavir tablets use. Advise patients to notify their healthcare provider if they develop the signs and symptoms of diabetes mellitus including frequent urination, excessive thirst, extreme hunger or unusual weight loss and/or an increased blood sugar while on lopinavir and ritonavir tablets as they may require a change in their diabetes treatment or new treatment [see Warnings and Precautions ( 5.7)].
Immune Reconstitution Syndrome
Advise patients that immune reconstitution syndrome has been reported in HIV-
infected patients treated with combination antiretroviral therapy, including
lopinavir and ritonavir tablets [see Warnings and Precautions (5.8)].
Lipid Disorders
Advise patients that treatment with lopinavir and ritonavir tablets therapy
can result in substantial increases in the concentration of total cholesterol
and triglycerides [see Warnings and Precautions (5.9)].
Fat Redistribution
Advise patients that redistribution or accumulation of body fat may occur in
patients receiving antiretroviral therapy and that the cause and long term
health effects of these conditions are not known at this time [see Warnings and Precautions ( 5.10)].
Patients with Hemophilia
Advise patients with hemophilia that they may experience increased bleeding
when treated with protease inhibitors such as lopinavir and ritonavir tablets
[see Warnings and Precautions (5.11)].
Pregnancy Exposure Registry
Inform patients that there is an antiretroviral pregnancy registry that
monitors fetal outcomes of pregnant women exposed to lopinavir and ritonavir
tablets [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:HETERO**TM**
****Hetero Labs Limited
Jeedimetla, Hyderabad – 500 055, India.
All brands listed are trademarks of their respective owners and are not trademarks of Hetero Labs Limited. The makers of these brands are not affiliated with and do not endorse Hetero Labs Limited or its products.
Revised: 02/2021
