Acetic Acid
0.25% Acetic Acid Irrigation, USPin Plastic Pour Bottle
41bc12af-fac5-4ac1-b907-dcb3e35a054c
HUMAN PRESCRIPTION DRUG LABEL
Feb 2, 2018
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acetic Acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
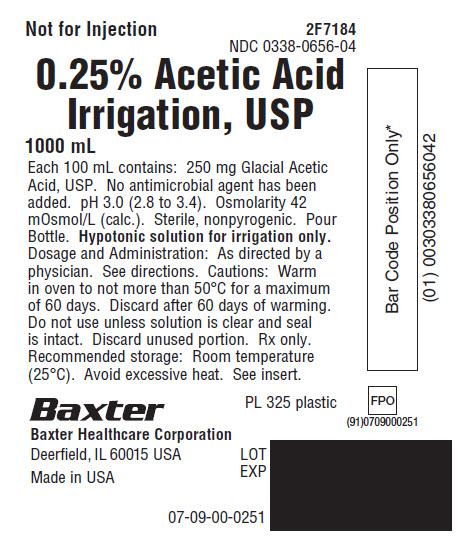
Container Label
Not for Injection 2F7184
NDC 0338-0656-04
0.25% Acetic Acid
Irrigation, USP
1000 mL
Each 100 mL contains: 250 mg Glacial Acetic
Acid, USP. No antimicrobial agent has been
added. pH 3.0 (2.8 to 3.4). Osmolarity 42
mOsmol/L (calc.). Sterile, nonpyrogenic. Pour
Bottle.Hypotonic solution for irrigation only.
****Dosage and Administration: As directed by a
physician. See directions. Cautions: Warm
in oven to not more than 50°C for a maximum
of 60 days. Discard after 60 days of warming.
Do not use unless solution is clear and seal
is intact. Discard unused portion. Rx only.
Recommended storage: Room temperature
(25°C). Avoid excessive heat. See insert.
PL 325 plastic
Baxter Logo****
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USA
LOT
EXP
07-09-00-0251
FPO
(91)0709000251
Bar Code
Bar Code Posit ion Only*
(01) 00303380656042
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
0.25% Acetic Acid Irrigation, USP, is indicated as a constant or intermittent bladder rinse to help prevent the growth and proliferation of susceptible urinary pathogens (especially ammonia forming bacteria) in the management of patients who require prolonged placement of an indwelling urethral catheter. It also may be used for periodic irrigation of an indwelling catheter to help maintain patency by reducing the formation of calcium encrustations.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
None known
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Systemic acidosis, pain and hematuria have been reported in patients receiving urinary bladder irrigation with 0.25% Acetic Acid Irrigation, USP.
Should adverse reactions occur, discontinue the irrigation and reevaluate the clinical status of the patient.
DESCRIPTION SECTION
DESCRIPTION
0.25% Acetic Acid Irrigation, USP, is a sterile, nonpyrogenic hypotonic solution for irrigation of the urinary bladder. Each 100 mL contains 250 mg Glacial Acetic Acid, USP, (CH3COOH) in Water for Injection, USP. pH 3.0 (2.8 to 3.4). Osmolarity: 42 mOsmol/L (calc.). No antimicrobial agent has been added.
The container is made from specially formulated polyolefin (PL 325). The polyolefin is a copolymer of ethylene and propylene. It contains no plasticizers or other mobile additives. As a result, the container has virtually no extractability or leachability. The total extractables after two years of storage being less than 0.01 ppm. It is also relatively impermeable to water vapor transmission and, therefore, requires no vapor barrier to maintain the proper drug concentration.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
The minimal amount of acetic acid which may enter the systemic circulation is readily metabolized.
WARNINGS SECTION
WARNINGS
Not for injection.
Use of this solution in patients with mucosal lesions of the urinary bladder may be harmful due to irritation of the lesion. Absorption via open lesions of the bladder mucosa may result in systemic acidosis.
The contents of an opened container should be used promptly to minimize the possibility of bacterial growth or pyrogen formation. Discard the unused portion of irrigating solution since no antimicrobial agent has been added.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
The volume of solution needed will vary with nature and duration of the urologic procedure, according to physician’s instructions.
Irrigation drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
SPL UNCLASSIFIED SECTION
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
Baxter is a registered trademark of Baxter International Inc.
07-19-00-0228
Rev. February 2018
PRECAUTIONS SECTION
PRECAUTIONS
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with 0.25% Acetic Acid Irrigation, USP. It is also not known whether 0.25% Acetic Acid Irrigation, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 0.25% Acetic Acid Irrigation, USP should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Warm in oven to not more than 50°C for a maximum of 60 days. Discard after 60 days of warming.
Do not administer unless the solution is clear and the seal is intact.
HOW SUPPLIED SECTION
HOW SUPPLIED
0.25% Acetic Acid Irrigation, USP is supplied in a plastic pour bottle as follows:
|
2F7184 |
1000 mL |
NDC 0338-0656-04 |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended that the product be stored at room temperature (25°C): brief exposure up to 40ºC does not adversely affect the product.
