SUPRANE
These highlights do not include all the information needed to use SUPRANE safely and effectively. See full prescribing information for SUPRANE. SUPRANE (desflurane) liquid, for inhalation useInitial U.S. Approval: 1992
3fe3d468-d283-4dd0-a1a7-29291a9b2d0b
HUMAN PRESCRIPTION DRUG LABEL
Feb 22, 2023
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
desflurane
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL

Container Label
Container Label
NDC 10019-641-60
Suprane
(desflurane, USP)
Liquid for Inhalation
240 mL
Rx only
Baxter
Manufactured for
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
3 1001964160 7
LOT
EXP. DATE
MFG DATE
(±) 1,2,2,2 - tetrafluoroethyl
difluoromethyl ether
A nonflammable, nonexplosive
inhalation anesthetic
Store at room temperature
15°-30°C(59°-86°F).
Replace cap after each use.
**IMPORTANT:**See package insert for
dosage and directions for use.
For Product Inquiry 1 800 ANA DRUG
(1-800-262-3784)
Baxter, Suprane and the Suprane bottle shape
are trademarks of Baxter International Inc.
07-09-69-714
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Contraindications (4) 11/2022
Warnings and Precautions; Malignant Hyperthermia (5.1) 11/2022
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Changes in the clinical effects of SUPRANE rapidly follow changes in the inspired concentration. The duration of anesthesia and selected recovery measures for SUPRANE are given in the following tables:
In 178 female outpatients undergoing laparoscopy, premedicated with fentanyl (1.5-2.0 µg/kg), anesthesia was initiated with propofol 2.5 mg/kg, desflurane/N2O 60% in O2 or desflurane/O2 alone. Anesthesia was maintained with either propofol 1.5-9.0 mg/kg/hr, desflurane 2.6-8.4% in N2O 60% in O2, or desflurane 3.1-8.9% in O2.
| ||||
|
Emergence and Recovery After Outpatient Laparoscopy | ||||
|
Induction: |
Propofol |
Propofol |
Desflurane/N2O |
Desflurane/O****2 |
|
Maintenance: |
Propofol/N2O |
Desflurane/N2O |
Desflurane/N2O |
Desflurane/O****2 |
|
Number of Pts: |
N = 48 |
N = 44 |
N = 43 |
N = 43 |
|
Median age |
30 |
26 |
29 |
30 |
|
Anesthetic time |
49 ± 53 |
45 ± 35 |
44 ± 29 |
41 ± 26 |
|
Time to open eyes |
7 ± 3 |
5 ± 2* |
5 ± 2* |
4 ± 2* |
|
Time to state name |
9 ± 4 |
8 ± 3 |
7 ± 3* |
7 ± 3* |
|
Time to stand |
80 ± 34 |
86 ± 55 |
81 ± 38 |
77 ± 38 |
|
Time to walk |
110 ± 6 |
122 ± 85 |
108 ± 59 |
108 ± 66 |
|
Time to fit for discharge |
152 ± 75 |
157 ± 80 |
150 ± 66 |
155 ± 73 |
In 88 unpremedicated outpatients, anesthesia was initiated with thiopental 3-9 mg/kg or desflurane in O2. Anesthesia was maintained with isoflurane 0.7-1.4% in N2O 60%, desflurane 1.8-7.7% in N2O 60%, or desflurane 4.4-11.9% in O2.
| ||||
|
Emergence and Recovery Times in Outpatient Surgery | ||||
|
Induction: |
Thiopental |
Thiopental |
Thiopental |
Desflurane/O****2 |
|
Maintenance: |
Isoflurane/N2O |
Desflurane/N2O |
Desflurane/O****2 |
Desflurane/O****2 |
|
Number of Pts: |
N = 23 |
N = 21 |
N = 23 |
N = 21 |
|
Median age |
43 |
40 |
43 |
41 |
|
Anesthetic time |
49 ± 23 |
50 ± 19 |
50 ± 27 |
51 ± 23 |
|
Time to open eyes |
13 ± 7 |
9 ± 3* |
12 ± 8 |
8 ± 2* |
|
Time to state name |
17 ± 10 |
11 ± 4* |
15 ± 10 |
9 ± 3* |
|
Time to walk |
195 ± 67 |
176 ± 60 |
168 ± 34 |
181 ± 42 |
|
Time to fit for discharge |
205 ± 53 |
202 ± 41 |
197 ± 35 |
194 ± 37 |
Recovery from anesthesia was assessed at 30, 60, and 90 minutes following 0.5 MAC desflurane (3%) or isoflurane (0.6%) in N2O 60% using subjective and objective tests. At 30 minutes after anesthesia, only 43% of patients in the isoflurane group were able to perform the psychometric tests compared to 76% in the SUPRANE group (p < 0.05).
| ||||
|
Recovery Tests: Percent of Preoperative Baseline Values | ||||
|
16 Males, 22 Females, Ages 20-65 Percent: Mean ± SD | ||||
|
60 minutes After Anesthesia |
90 minutes After Anesthesia | |||
|
Maintenance: |
** Desflurane/N2O** |
** Isoflurane/N2O** |
** Desflurane/N2O** |
** Isoflurane/N2O** |
|
Confusion* |
66 ± 6 |
47 ± 8 |
75 ± 7† |
56 ± 8 |
|
Fatigue* |
70 ± 9† |
33 ± 6 |
89 ± 12† |
47 ± 8 |
|
Drowsiness* |
66 ± 5† |
36 ± 8 |
76 ± 7† |
49 ± 9 |
|
Clumsiness* |
65 ± 5 |
49 ± 8 |
80 ± 7† |
57 ± 9 |
|
Comfort* |
59 ± 7† |
30 ± 6 |
60 ± 8† |
31 ± 7 |
|
DSST‡** score** |
74 ± 4† |
50 ± 9 |
75 ± 4† |
55 ± 7 |
|
Trieger Tests§ |
67 ± 5 |
74 ± 6 |
90 ± 6 |
83 ± 7 |
SUPRANE was studied in twelve volunteers receiving no other drugs. Hemodynamic effects during controlled ventilation (PaCO2 38 mm Hg) were:
| ||||||||
|
Hemodynamic Effects of Desflurane During Controlled Ventilation | ||||||||
|
12 Male Volunteers, Ages 16-26 | ||||||||
|
Heart Rate |
Mean Arterial ** (mm Hg)** |
Cardiac Index | ||||||
|
Total |
End-Tidal % Des/O****2 |
End-Tidal % Des/N2O |
O****2 |
N2O |
O****2 |
N2O |
O****2 |
N2O |
|
0 |
0% / 21% |
0% / 0% |
69 ± 4 |
70 ± 6 |
85 ± 9 |
85 ± 9 |
3.7 ± 0.4 |
3.7 ± 0.4 |
|
0.8 |
6% / 94% |
3% / 60% |
73 ± 5 |
77 ± 8 |
61 ± 5* |
69 ± 5* |
3.2 ± 0.5 |
3.3 ± 0.5 |
|
1.2 |
9% / 91% |
6% / 60% |
80 ± 5* |
77 ± 7 |
59 ± 8* |
63 ± 8* |
3.4 ± 0.5 |
3.1 ± 0.4* |
|
1.7 |
12% / 88% |
9% / 60% |
94 ± 14* |
79 ± 9 |
51 ± 12* |
59 ± 6* |
3.5 ± 0.9 |
3.0 ± 0.4* |
When the same volunteers breathed spontaneously during desflurane anesthesia, systemic vascular resistance and mean arterial blood pressure decreased; cardiac index, heart rate, stroke volume, and central venous pressure (CVP) increased compared to values when the volunteers were conscious. Cardiac index, stroke volume, and CVP were greater during spontaneous ventilation than during controlled ventilation.
During spontaneous ventilation in the same volunteers, increasing the concentration of SUPRANE from 3% to 12% decreased tidal volume and increased arterial carbon dioxide tension and respiratory rate. The combination of N2O 60% with a given concentration of desflurane gave results similar to those with desflurane alone. Respiratory depression produced by desflurane is similar to that produced by other potent inhalation agents.
The use of desflurane concentrations higher than 1.5 MAC may produce apnea.
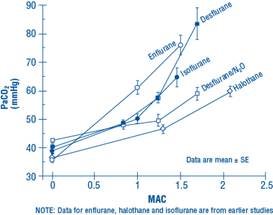
Figure 1. PaCO2 During Spontaneous Ventilation in Unstimulated Volunteers
12.3 Pharmacokinetics
Due to the volatile nature of desflurane in plasma samples, the washin-washout profile of desflurane was used as a surrogate of plasma pharmacokinetics. SUPRANE is a volatile liquid inhalation anesthetic minimally biotransformed in the liver in humans. Less than 0.02% of the desflurane absorbed can be recovered as urinary metabolites (compared to 0.2% for isoflurane). Eight healthy male volunteers first breathed 70% N2O/30% O2 for 30 minutes and then a mixture of desflurane 2.0%, isoflurane 0.4%, and halothane 0.2% for another 30 minutes. During this time, inspired and end-tidal concentrations (FI and FA) were measured. The FA/FI (washin) value at 30 minutes for desflurane was 0.91, compared to 1.00 for N2O, 0.74 for isoflurane, and 0.58 for halothane (see Figure 2). The washin rates for halothane and isoflurane were similar to literature values. The washin was faster for desflurane than for isoflurane and halothane at all time points. The FA/FAO (washout) value at 5 minutes was 0.12 for desflurane, 0.22 for isoflurane, and 0.25 for halothane (see Figure 3). The washout for desflurane was more rapid than that for isoflurane and halothane at all elimination time points. By 5 days, the FA/FAO for desflurane is 1/20th of that for halothane or isoflurane.
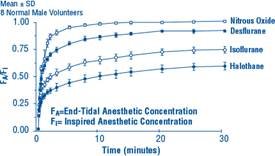
Figure 2. Desflurane Washin
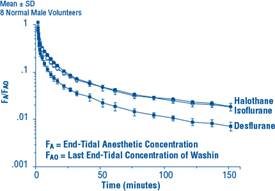
Figure 3. Desflurane Washout
12.5 Pharmacogenomics
RYR1 and CACNA1S are polymorphic genes and multiple pathogenic variants have been associated with malignant hyperthermia susceptibility (MHS) in patients receiving volatile anesthetic agents, including SUPRANE. Case reports as well as ex-vivo studies have identified multiple variants in RYR1 and CACNA1S associated with MHS. Variant pathogenicity should be assessed based on prior clinical experience, functional studies, prevalence information, or other evidence [see Contraindications (4), Warnings and Precautions (5.1)].
DESCRIPTION SECTION
11 DESCRIPTION
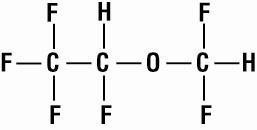
SUPRANE (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is (±)1,2,2,2-tetrafluoroethyl difluoromethyl ether:
Some physical constants are:
|
168.04 |
|
1.465 |
|
669 mm Hg @ 20°C |
|
731 mm Hg @ 22°C | |
|
757 mm Hg @ 22.8°C (boiling point;1atm) | |
|
764 mm Hg @ 23°C | |
|
798 mm Hg @ 24°C | |
|
869 mm Hg @ 26°C |
Partition coefficients at 37°C:
|
0.424 |
|
18.7 |
|
0.54 |
Mean Component/Gas Partition Coefficients:
|
6.7 |
|
16.2 |
|
19.3 |
|
10.4 |
|
34.7 |
SUPRANE is nonflammable as defined by the requirements of International Electrotechnical Commission 601-2-13.
SUPRANE is a colorless, volatile liquid below 22.8°C. Data indicate that SUPRANE is stable when stored under normal room lighting conditions according to instructions.
SUPRANE is chemically stable. The only known degradation reaction is through prolonged direct contact with soda lime producing low levels of fluoroform (CHF3). The amount of CHF3 obtained is similar to that produced with MAC- equivalent doses of isoflurane. No discernible degradation occurs in the presence of strong acids.
SUPRANE does not corrode stainless steel, brass, aluminum, anodized aluminum, nickel plated brass, copper, or beryllium.
