Stance
Stance 0.63% Stannous Fluoride Concentrate Rinse Mint
3ae71258-a8c2-6eb5-e054-00144ff88e88
HUMAN OTC DRUG LABEL
Sep 22, 2025
Elevate Oral Care
DUNS: 002863526
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
0.63% Stannous Fluoride Concentrate Rinse
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
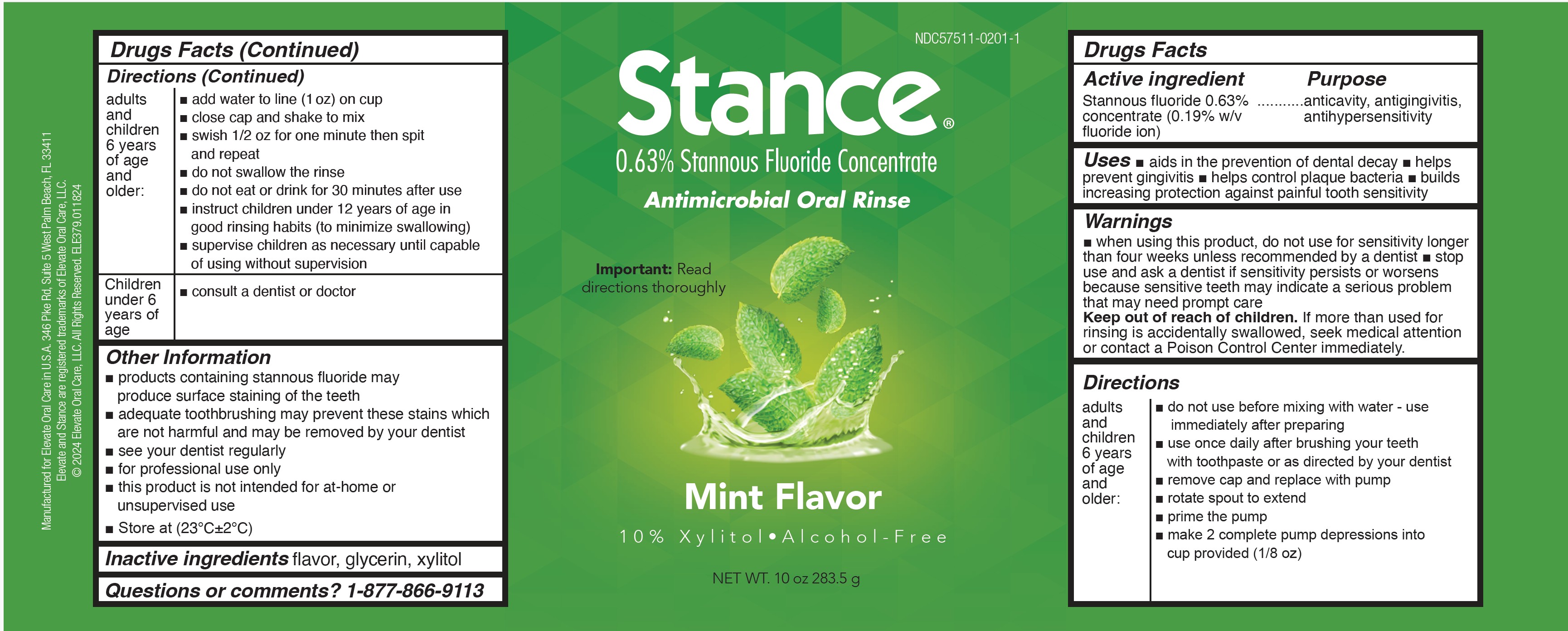
INDICATIONS & USAGE SECTION
Uses
INDICATIONS & USAGE SECTION

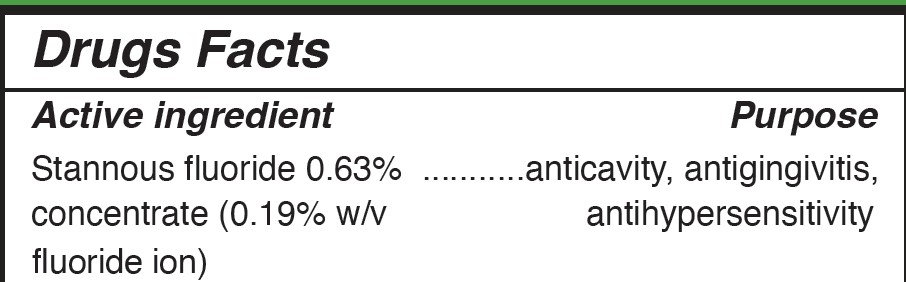
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENT
WARNINGS SECTION
WARNINGS

OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out of Reach of Children

INSTRUCTIONS FOR USE SECTION
Directions
INSTRUCTIONS FOR USE SECTION

INACTIVE INGREDIENT SECTION
Inactive Ingredients

OTC - QUESTIONS SECTION
Questions?

DOSAGE & ADMINISTRATION SECTION
Dosage and Administration
make 2 complete pump depressions into cup
provided (1/8 oz)
• add tap water to line (1 oz) on cup
• close cap and shake to mix
• vigorously swish 1/2 oz for one minute then
spit out and repeat with second 1/2 oz
• do not swallow the rinse
OTC - PURPOSE SECTION
Purpose

OTHER SAFETY INFORMATION
Other Information

