Hydrocortisone
Hydrocortisone tablets, USP
bc751403-94f2-4f9d-b533-cf6186a40ceb
HUMAN PRESCRIPTION DRUG LABEL
Jan 23, 2024
Greenstone LLC
DUNS: 825560733
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
hydrocortisone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
hydrocortisone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
hydrocortisone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label
NDC 59762-0075-1
100 Tablets
GREENSTONE® BRAND
hydrocortisone
tablets, USP
20 mg
Rx only

WARNINGS SECTION
WARNINGS
In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
Immunosuppression and Increased Risk of Infection
Corticosteroids, including CORTEF, suppress the immune system and increase the risk of
infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic
pathogens. Corticosteroids can:
•
Reduce resistance to new infections
•
Exacerbate existing infections
•
Increase the risk of disseminated infections
•
Increase the risk of reactivation or exacerbation of latent infections
•
Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The
rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider CORTEF withdrawal or dosage
reduction as needed.
Tuberculosis
If CORTEF is used to treat a condition in patients with latent tuberculosis or tuberculin
reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for
reactivation. During prolonged CORTEF therapy, patients with latent tuberculosis or
tuberculin reactivity should receive chemoprophylaxis.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients
taking corticosteroids, including CORTEF. In corticosteroid-treated patients who have
not had these diseases or are non-immune, particular care should be taken to avoid
exposure to varicella and measles:
•
If a CORTEF-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
•
If a CORTEF-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including CORTEF. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with CORTEF. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including CORTEF, may exacerbate systemic fungal infections; therefore, avoid CORTEF use in the presence of such infections unless CORTEF is needed to control drug reactions. For patients on chronic CORTEF therapy who develop systemic fungal infections, CORTEF withdrawal or dosage reduction is recommended.
Amebiasis
Corticosteroids, including CORTEF, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating CORTEF in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including CORTEF, should be used with great care in patients with
known or suspected Strongyloides (threadworm) infestation. In such patients,
corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and
dissemination with widespread larval migration, often accompanied by severe
enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria
Avoid corticosteroids, including CORTEF, in patients with cerebral malaria.
Ophthalmic Effects
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Kaposi’s Sarcoma
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Hypertension, Volume Overload, and Hypokalemia
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Vaccinations
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered to patients receiving immunosuppressive doses of corticosteroids; however, the response to such vaccines may be diminished. Indicated immunization procedures may be undertaken in patients receiving nonimmunosuppressive doses of corticosteroids.
Usage in Pregnancy
Since adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers or women of child bearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy, should be carefully observed for signs of hypoadrenalism.
Corticosteroids have been shown to impair fertility in male rats.
PRECAUTIONS SECTION
PRECAUTIONS
General Precautions
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted.
There is an enhanced effect of corticosteroids on patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition under treatment, and when reduction in dosage is possible, the reduction should be gradual.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability of impending perforation, abscess or other pyogenic infection; diverticulitis; fresh intestinal anastomoses; active or latent peptic ulcer; renal insufficiency; hypertension; osteoporosis; and myasthenia gravis.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
Pheochromocytoma crisis, which can be fatal, has been reported after administration of systemic corticosteroids. In patients with suspected pheochromocytoma, consider the risk of pheochromocytoma crisis prior to administering corticosteroids.
In post marketing experience tumor lysis syndrome (TLS) has been reported in patients with malignancies, including hematological malignancies and solid tumors, following the use of systemic corticosteroids alone or in combination with other chemotherapeutic agents. Patients at high risk of TLS, such as patients with tumors that have a high proliferative rate, high tumor burden and high sensitivity to cytotoxic agents, should be monitored closely and appropriate precautions should be taken.
Drug Interactions
The pharmacokinetic interactions listed below are potentially clinically important. Drugs that induce hepatic enzymes such as phenobarbital, phenytoin and rifampin may increase the clearance of corticosteroids and may require increases in corticosteroid dose to achieve the desired response. Drugs such as troleandomycin and ketoconazole may inhibit the metabolism of corticosteroids and thus decrease their clearance. Therefore, the dose of corticosteroid should be titrated to avoid steroid toxicity. Corticosteroids may increase the clearance of chronic high dose aspirin. This could lead to decreased salicylate serum levels or increase the risk of salicylate toxicity when corticosteroid is withdrawn. Aspirin should be used cautiously in conjunction with corticosteroids in patients suffering from hypoprothrombinemia. The effect of corticosteroids on oral anticoagulants is variable. There are reports of enhanced as well as diminished effects of anticoagulants when given concurrently with corticosteroids. Therefore, coagulation indices should be monitored to maintain the desired anticoagulant effect.
Information for the Patient
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
DESCRIPTION SECTION
DESCRIPTION
Hydrocortisone tablets contain hydrocortisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Hydrocortisone USP is white to practically white, odorless, crystalline powder with a melting point of about 215° C. It is very slightly soluble in water and in ether; sparingly soluble in acetone and in alcohol; slightly soluble in chloroform.
The chemical name for hydrocortisone is pregn-4-ene-3,20-dione,11,17,21-trihydroxy-, (11β)-. Its molecular weight is 362.46 and the structural formula is as outlined below.
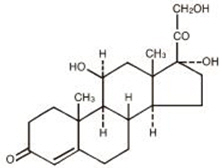
Hydrocortisone tablets are available for oral administration in three strengths: each tablet contains either 5 mg, 10 mg, or 20 mg of hydrocortisone. Inactive ingredients: calcium stearate, corn starch, lactose, mineral oil, sorbic acid, sucrose.
SPL UNCLASSIFIED SECTION

LAB-0634-6.0
Revised September 2024
