Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
079637826
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Whole Foods Market, INC.
Bionpharma Inc.
675584180
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nighttime Sleep-Aid
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
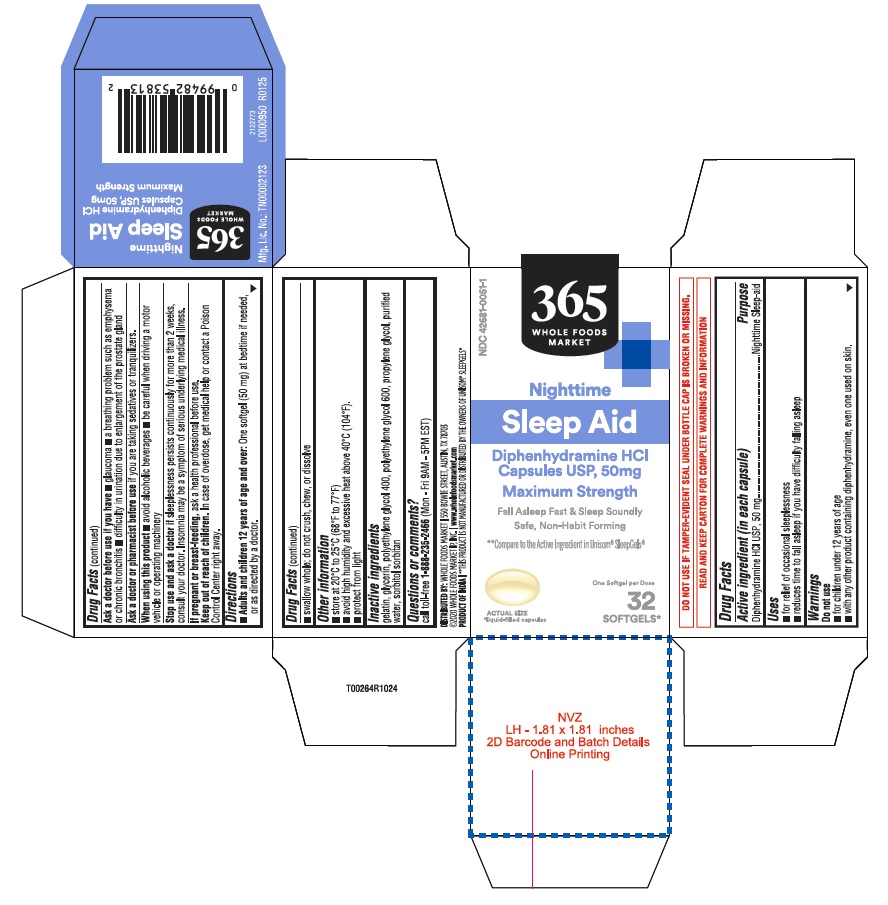
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
32s count
NDC 42681-0051-1
365
WHOLE FOODS
MARKET
Nighttime
Sleep-Aid
Diphenhydramine HCl
Capsules USP, 50 mg
Maximum Strength
Fall asleep fast & Sleep Soundly
safe, non habit-forming
Compare to the Active Ingredient in****Unisom®**SleepGels®
One softgel per dose
32
SOFTGELS*
*Liquid-filled capsules
INDICATIONS & USAGE SECTION
Uses
■ for relief of occasional sleeplessness ■ reduces time to fall asleep if you have difficulty falling asleep
DOSAGE & ADMINISTRATION SECTION
Directions
■**Adults and children 12 years of age and over:**One softgel (50 mg) at
bedtime if needed, or as directed by a doctor.
■ swallow whole; do not crush, chew, or dissolve
WARNINGS SECTION
Warnings
Do not use
■ for children under 12 years of age
■ with any other product containing diphenhydramine, even one used on skin.
STORAGE AND HANDLING SECTION
Other information
■ store at 20°C to 25°C (68°F to 77°F) ■ avoid high humidity and excessive
heat above 40°C (104°F).
■ protect from light
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each capsule)
Diphenhydramine HCl 50 mg
OTC - PURPOSE SECTION
Purpose
Nighttime sleep-aid
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
■ glaucoma ■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate gland
OTC - ASK DOCTOR/PHARMACIST SECTION
Ask a doctor or pharmacist before use
if you are taking sedatives or tranquilizers.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
OTC - STOP USE SECTION
Stop use and ask a doctor if
sleeplessness persists continuosly for more than 2 weeks, consult your doctor. Insomnia may be a symptom of serious underlying medical illness.
OTC - WHEN USING SECTION
When using this product
■ avoid alcoholic beverages ■ be careful when driving a motor vehicle or operating machinery
OTC - QUESTIONS SECTION
Questions or comments?
call toll-free1-888-235-2466 (Mon - Fri 9AM - 5PM EST)
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast- feeding,
ask a health professional before use.
SPL UNCLASSIFIED SECTION
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IS BROKEN OR MISSING.
READ AND KEEP CARTON FOR COMPLETE WARNINGS AND INFORMATION
DISTRIBUTED BY: WHOLE FOODS MARKET I 550 BOWIE STREET, AUSTIN, TX 78703
©2020 WHOLE FOODS MARKET IP, INC. I www.wholefoodsmarket.com
PRODUCT OF INDIA I**THIS PRODUCT IS NOT MANUFACTURED OR DISIRIBUTED BY THE
OWNERS OF UNISOM® SLEEPGELS®
L0000950 R0125
Mfg. Lic. No.: TN00002123
Lot No.:
Exp. Date:
INACTIVE INGREDIENT SECTION
Inactive ingredients
gelatin, glycerin, polyethylene glycol 400, polyethylene glycol 600, propylene glycol, purified water, sorbitol sorbitan solution
