Minocycline Hydrochloride
These highlights do not include all the information needed to use MINOCYCLINE HYDROCHLORIDE EXTENDED RELEASE TABLETS safely and effectively. See full prescribing information for MINOCYCLINE HYDROCHLORIDE EXTENDED RELEASE TABLETS. MINOCYCLINE HYDROCHLORIDE extended-release tablets, for oral use Initial U.S. Approval: 1971
9c43389b-dc98-41f3-80bf-73d916ad6843
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Minocycline Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Minocycline HCL 115 mg ER tab#30

Description Section
11 DESCRIPTION
Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is
[4S-(4α,4aα,5aα, 12aα)] - 4,7 – Bis (dimethylamino) -1,4,4a,5,5a,6,11,12a -
octahydro-3,10,12,12a -tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono
hydrochloride.
The structural formula is represented below:
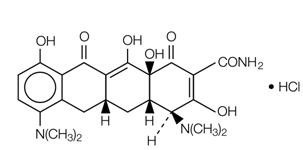
C23H27N3O7•HCl M. W. 493.95
Minocycline hydrochloride extended-release tablets, USP for oral administration contain minocycline hydrochloride USP equivalent to 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, and 135 mg of minocycline. In addition, 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, and 135 mg tablets contain the following inactive ingredients: lactose monohydrate, hypromellose type 2910, hypromellose type 2208, colloidal silicon dioxide, magnesium stearate, titanium dioxide and triacetin.
The 45 mg tablets also contain iron oxide black.
The 65 mg tablets also contain FD&C blue #1/brilliant blue FCF aluminium lake,
polyethylene glycol 3350, FD&C blue #2/indigo carmine aluminum lake and D&C yellow #10 aluminum lake.
The 55 mg tablets also contain macrogol, FD&C RED #40.
The 80 mg tablets also contain macrogol, FD&C blue #2, FD&C red #40, FD&C yellow
#6.
The 90 mg tablets also contain iron oxide yellow and polyethylene glycol 3350.
The 105 mg tablets also D&C red #27, macrogol, FD&C blue #1.
The 115 mg tablets also contain D&C yellow #10 aluminum lake, FD&C blue #1/brilliant blue FCF aluminium lake and FD&C blue #2/indigo carmine aluminum lake.
The 135 mg tablets also contain polyethylene glycol 3350 and iron oxide red.
The USP Dissolution Test is pending
How Supplied Section
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Minocycline hydrochloride extended-release tablets, USP are supplied as
aqueous film coated tablets containing Minocycline hydrochloride equivalent to
115 mg minocycline, are supplied as follows.
The 115 mg extended release tablets are green coloured capsule shaped film coated tablets, debossed with 115 on one side, plain on other side. Each tablet contains Minocycline hydrochloride equivalent to 115 mg minocycline, supplied as follows:
|
NDC 63629-9209-1 |
Bottle of 30 |
16.2 Storage
Store at 20˚ to 25˚C (68˚ to 77˚ F) [See USP Controlled Room Temperature].
16.3 Handling
Keep out of reach of children.
Protect from light, moisture, and excessive heat.
Dispense in tight, light-resistant container with child-resistant closure.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
