Nilutamide
Nilutamide Tablets
34ea4810-2d6c-4bd9-ae52-8bab786dee91
HUMAN PRESCRIPTION DRUG LABEL
Dec 10, 2021
Prasco Laboratories
DUNS: 065969375
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nilutamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Nilutamide tablets contain nilutamide, a nonsteroidal, orally active antiandrogen having the chemical name 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione with the following structural formula:
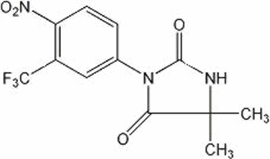
Nilutamide is a microcrystalline, white to practically white powder with a molecular weight of 317.25. Its molecular formula is C12H10F3N3O4.
It is freely soluble in ethyl acetate, acetone, chloroform, ethyl alcohol, dichloromethane, and methanol. It is slightly soluble in water [<0.1% W/V at 25°C (77°F)]. It melts between 153°C and 156°C (307.4°F and 312.8°F).
Each Nilutamide tablet contains 150 mg of nilutamide. Other ingredients in Nilutamide tablets are corn starch, lactose, povidone, docusate sodium, magnesium stearate, and talc.
