ISENTRESS
These highlights do not include all the information needed to use ISENTRESS safely and effectively. See full prescribing information for ISENTRESS. ISENTRESS (raltegravir) film-coated tablets, for oral useISENTRESS HD (raltegravir) film-coated tablets, for oral useISENTRESS (raltegravir) chewable tablets, for oral useISENTRESS (raltegravir) for oral suspensionInitial U.S. Approval: 2007
7df185ff-4347-4155-939c-09288f3ebbd4
HUMAN PRESCRIPTION DRUG LABEL
Jul 4, 2023
RPK Pharmaceuticals, Inc.
DUNS: 147096275
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Adult Patients:
ISENTRESS® and ISENTRESS® HD are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in adult patients.
Pediatric Patients:
ISENTRESS is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in pediatric patients weighing at least 2 kg.
ISENTRESS HD is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in pediatric patients weighing at least 40 kg.
Adult Patients:
ISENTRESS and ISENTRESS HD are human immunodeficiency virus integrase strand transfer inhibitors (HIV-1 INSTI) indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients (1).
Pediatric Patients:
ISENTRESS is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in pediatric patients weighing at least 2 kg (1).
ISENTRESS HD is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in pediatric patients weighing at least 40 kg (1).
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Severe Skin and Hypersensitivity Reactions
Severe, potentially life-threatening, and fatal skin reactions have been reported. These include cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Hypersensitivity reactions have also been reported and were characterized by rash, constitutional findings, and sometimes, organ dysfunction, including hepatic failure. Discontinue ISENTRESS or ISENTRESS HD and other suspect agents immediately if signs or symptoms of severe skin reactions or hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, hepatitis, eosinophilia, angioedema). Clinical status including liver aminotransferases should be monitored and appropriate therapy initiated. Delay in stopping ISENTRESS or ISENTRESS HD treatment or other suspect agents after the onset of severe rash may result in a life-threatening reaction.
5.2 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including ISENTRESS. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia, tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain- Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.3 Phenylketonurics
ISENTRESS Chewable Tablets contain phenylalanine, a component of aspartame. Each 25 mg ISENTRESS Chewable Tablet contains approximately 0.05 mg phenylalanine. Each 100 mg ISENTRESS Chewable Tablet contains approximately 0.10 mg phenylalanine. Phenylalanine can be harmful to patients with phenylketonuria.
- Severe, potentially life-threatening and fatal skin reactions have been reported. This includes cases of Stevens-Johnson syndrome, hypersensitivity reaction and toxic epidermal necrolysis. Immediately discontinue treatment with ISENTRESS or ISENTRESS HD and other suspect agents if severe hypersensitivity, severe rash, or rash with systemic symptoms or liver aminotransferase elevations develops and monitor clinical status, including liver aminotransferases closely (5.1).
- Monitor for Immune Reconstitution Syndrome (5.2).
- Inform patients with phenylketonuria that the 100 mg and 25 mg chewable tablets contain phenylalanine (5.3).
DESCRIPTION SECTION
11 DESCRIPTION
ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl) methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide monopotassium salt.
The empirical formula is C20H20FKN6O5 and the molecular weight is 482.51. The structural formula is:
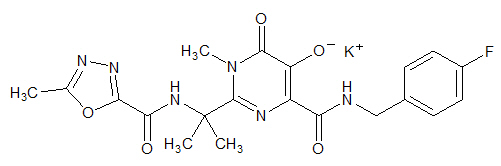
Raltegravir potassium is a white to off-white powder. It is soluble in water, slightly soluble in methanol, very slightly soluble in ethanol and acetonitrile and insoluble in isopropanol.
Each 400 mg film-coated tablet of ISENTRESS for oral administration contains 434.4 mg of raltegravir (as potassium salt), equivalent to 400 mg of raltegravir free phenol and the following inactive ingredients: calcium phosphate dibasic anhydrous, hypromellose 2208, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: black iron oxide, polyethylene glycol 3350, polyvinyl alcohol, red iron oxide, talc and titanium dioxide.
Each 600 mg film-coated tablet of ISENTRESS HD for oral administration contains 651.6 mg of raltegravir (as potassium salt), equivalent to 600 mg of raltegravir free phenol and the following inactive ingredients: croscarmellose sodium, hypromellose 2910, magnesium stearate, microcrystalline cellulose. The film coating contains the following inactive ingredients: ferrosoferric oxide, hypromellose 2910, iron oxide yellow, lactose monohydrate, triacetin and titanium dioxide. The tablet may also contain trace amount of carnauba wax.
Each 100 mg chewable tablet of ISENTRESS for oral administration contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, red iron oxide, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each 25 mg chewable tablet of ISENTRESS for oral administration contains 27.16 mg of raltegravir (as potassium salt), equivalent to 25 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each packet of ISENTRESS for oral suspension 100 mg, contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, banana with other natural flavors, carboxymethylcellulose sodium, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, macrogol/PEG 400, magnesium stearate, maltodextrin, mannitol, medium chain triglycerides, microcrystalline cellulose, monoammonium glycyrrhizinate, oleic acid, sorbitol, sucralose and sucrose.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies of raltegravir in mice did not show any carcinogenic potential. At the highest dose levels, 400 mg/kg/day in females and 250 mg/kg/day in males, systemic exposure was 1.8-fold (females) or 1.2-fold (males) greater than the AUC (54 µM∙hr) at the 400-mg twice daily human dose. Treatment-related squamous cell carcinoma of nose/nasopharynx was observed in female rats dosed with 600 mg/kg/day raltegravir for 104 weeks. These tumors were possibly the result of local irritation and inflammation due to local deposition and/or aspiration of drug in the mucosa of the nose/nasopharynx during dosing. No tumors of the nose/nasopharynx were observed in rats dosed with 150 mg/kg/day (males) and 50 mg/kg/day (females) and the systemic exposure in rats was 1.7-fold (males) to 1.4-fold (females) greater than the AUC (54 µM∙hr) at the 400-mg twice daily human dose.
No evidence of mutagenicity or genotoxicity was observed in in vitro microbial mutagenesis (Ames) tests, in vitro alkaline elution assays for DNA breakage, and in vitro and in vivo chromosomal aberration studies.
No effect on fertility was seen in male and female rats at doses up to 600 mg/kg/day which resulted in a 3-fold exposure above the exposure at the recommended human dose.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Severe and Potentially Life-threatening Rash
Inform patients that severe and potentially life-threatening rash has been reported. Advise patients to immediately contact their healthcare provider if they develop rash. Instruct patients to immediately stop taking ISENTRESS or ISENTRESS HD and other suspect agents, and seek medical attention if they develop a rash associated with any of the following symptoms as it may be a sign of a more serious reaction such as Stevens-Johnson syndrome, toxic epidermal necrolysis or severe hypersensitivity: fever, generally ill feeling, extreme tiredness, muscle or joint aches, blisters, oral lesions, eye inflammation, facial swelling, swelling of the eyes, lips, mouth, breathing difficulty, and/or signs and symptoms of liver problems (e.g., yellowing of the skin or whites of the eyes, dark or tea colored urine, pale colored stools/bowel movements, nausea, vomiting, loss of appetite, or pain, aching or sensitivity on the right side below the ribs). Inform patients that if severe rash occurs, their physician will closely monitor them, order laboratory tests and initiate appropriate therapy.
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.2)].
Rhabdomyolysis
Before patients begin ISENTRESS or ISENTRESS HD, ask them if they have a history of rhabdomyolysis, myopathy or increased creatine kinase or if they are taking medications known to cause these conditions such as statins, fenofibrate, gemfibrozil or zidovudine [see Adverse Reactions (6.1)].
Instruct patients to immediately report to their healthcare provider any unexplained muscle pain, tenderness, or weakness while taking ISENTRESS.
Phenylketonuria
Alert patients with phenylketonuria that ISENTRESS Chewable Tablets contain phenylalanine [see Warnings and Precautions (5.3)].
Drug Interactions
Inform patients that ISENTRESS or ISENTRESS HD may interact with some drugs and ask them to identify all their current medications including over-the- counter agents [see Drug Interactions (7.2)].
Missed Dosage
Inform patients that it is important to take ISENTRESS or ISENTRESS HD on a regular dosing schedule as instructed by their healthcare provider and to avoid missing doses as it can result in development of resistance [see Dosage and Administration (2)].
Important Administration Instructions
Film-Coated Tablets
Inform patients that the film-coated tablets must be swallowed whole.
Oral Suspension
Instruct parents and/or caregivers to read the Instructions for Use before preparing and administering ISENTRESS for oral suspension to pediatric patients. Instruct parents and/or caregivers that ISENTRESS for oral suspension should be administered within 30 minutes of mixing.
Chewable Tablets
Inform parents and/or caregivers that the chewable tablets can be chewed or swallowed whole. Additionally, the 25 mg chewable tablet may be crushed. Instruct parents and/or caregivers that ISENTRESS 25 mg chewable tablets, if crushed, should be administered immediately [see Dosage and Administration (2.1)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ISENTRESS or ISENTRESS HD during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see Use in Specific Populations (8.2)].
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
- Because the formulations have different pharmacokinetic profiles, do not substitute ISENTRESS chewable tablets or ISENTRESS for oral suspension for the ISENTRESS 400 mg film-coated tablet or the ISENTRESS HD 600 mg film-coated tablet. See specific dosing guidance for chewable tablets and the formulation for oral suspension.
- Because the extent to which ISENTRESS may be dialyzable is unknown, dosing before a dialysis session should be avoided [see Clinical Pharmacology (12.3)].
- ISENTRESS film-coated tablets must be swallowed whole.
- ISENTRESS chewable tablets may be chewed or swallowed whole. Maximum daily dose is 300 mg taken by mouth twice daily.
- For children who have difficulty chewing the 25 mg chewable tablet, the tablet may be crushed.
- Preparation of the crushed 25 mg chewable tablet:
- Place the tablet(s) in a small, clean cup. For each tablet, add a teaspoonful (~5 mL) of liquid (for example, water, juice, or breast milk).
- Within 2 minutes, the tablet(s) will absorb the liquid and fall apart.
- Using a spoon, crush any remaining pieces of the tablet(s). Immediately administer the entire dose orally.
- If any portion of the dose is left in the cup, add another teaspoonful (~5 mL) of liquid, swirl and administer immediately.
- Preparation of the crushed 25 mg chewable tablet:
- ISENTRESS for oral suspension:
- SeeInstructions for Use for details on preparation and administration of ISENTRESS for oral suspension.
- Using the provided mixing cup, combine 10 mL of water and the entire contents of one packet of ISENTRESS for oral suspension and mix. Each single-use packet for oral suspension contains 100 mg of raltegravir which is suspended in 10 mL of water giving a final concentration of 10 mg per mL. Maximum daily dose is 100 mg taken by mouth twice daily.
- Gently swirl the mixing cup for 45 seconds in a circular motion to mix the powder into a uniform suspension.Do not shake.
- Once mixed, measure the prescribed dose volume of suspension with a syringe and administer the dose orally. The dose should be administered orally within 30 minutes of mixing.
- Discard any remaining suspension into the trash.
2.2 Adults
The recommended adult dosage of ISENTRESS film-coated tablets is displayed in Table 1. ISENTRESS and ISENTRESS HD should be taken by mouth and may be taken with or without food [see Clinical Pharmacology (12.3)].
Table 1: Dosing Recommendations for ISENTRESS and ISENTRESS HD in Adult Patients|
Population |
Recommended Dose |
|---|---|
|
Treatment-naïve patients or patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily |
1200 mg (2 × 600 mg) once daily |
|
Treatment-experienced |
400 mg twice daily |
|
Treatment-naïve or treatment-experienced when coadministered with rifampin [see Drug Interactions (7.1)] |
800 mg (2 × 400 mg) twice daily |
2.3 Pediatrics
The recommended pediatric dosage of ISENTRESS is displayed in Table 2. ISENTRESS film-coated tablets, chewable tablets and for oral suspension should be taken by mouth and may be taken with or without food [see Clinical Pharmacology (12.3)].
Table 2: Dosing Recommendations for ISENTRESS and ISENTRESS HD in Pediatric Patients|
Recommended Pediatric Dosage and Formulation | ||||
|---|---|---|---|---|
|
Population/Weight |
Film-Coated Tablets 400 mg |
Film-Coated Tablets 600 mg |
Chewable Tablets 100 mg and 25 mg |
For Oral Suspension 100 mg |
| ||||
|
If at least 40 kg and either:
|
400 mg twice daily |
1200 mg (2 × 600 mg) once daily |
300 mg twice daily (see Table 3) |
NA |
|
If at least 25 kg |
400 mg twice daily* |
NA |
Weight-based dosing twice daily (see Table 3) |
NA |
|
If at least 4 weeks of age and weighing 3 kg to less than 25 kg |
NA |
NA |
Weight-based dosing twice daily (see Table 4) |
Weight-based dosing twice daily up to 20 kg (see Table 4) |
|
From birth to 4 weeks (28 days) weighing at least 2 kg |
NA |
NA |
NA |
Weight-based dosing once daily or twice daily (see Table 5) |
|
Body Weight |
Dose |
Number of 100 mg Chewable Tablets |
|---|---|---|
| ||
|
25 to less than 28 |
150 mg twice daily |
1.5 × 100 mg† twice daily |
|
28 to less than 40 |
200 mg twice daily |
2 × 100 mg twice daily |
|
At least 40 |
300 mg twice daily |
3 × 100 mg twice daily |
|
Body Weight |
Volume (Dose) of Suspension to be Administered |
Number of Chewable Tablets† |
|---|---|---|
| ||
|
3 to less than 4 |
2.5 mL (25 mg) twice daily |
1 × 25 mg twice daily‡ |
|
4 to less than 6 |
3 mL (30 mg) twice daily | |
|
6 to less than 8 |
4 mL (40 mg) twice daily |
2 × 25 mg twice daily‡ |
|
8 to less than 10 |
6 mL (60 mg) twice daily | |
|
10 to less than 14 |
8 mL (80 mg) twice daily |
3 × 25 mg twice daily‡ |
|
14 to less than 20 |
10 mL (100 mg) twice daily |
1 × 100 mg twice daily |
|
20 to less than 25 |
Not applicable |
1.5 × 100 mg§ twice daily |
- For full-term neonates (birth to 4 weeks [28 days] of age): Weight-based dosing of the oral suspension as specified in Table 5.
- No data are available in pre-term neonates. The use of ISENTRESS is not recommended in pre-term neonates.
|
Body Weight |
Volume (Dose) of Suspension to be Administered |
|---|---|
|
Note: If the mother has taken ISENTRESS or ISENTRESS HD 2-24 hours before delivery, the neonate's first dose should be given between 24-48 hours after birth. | |
| |
|
Birth to 1 Week - Once daily dosing***** | |
|
2 to less than 3 |
0.4 mL (4 mg) once daily |
|
3 to less than 4 |
0.5 mL (5 mg) once daily |
|
4 to less than 5 |
0.7 mL (7 mg) once daily |
|
1 to 4 Weeks - Twice daily dosing**†** | |
|
2 to less than 3 |
0.8 mL (8 mg) twice daily |
|
3 to less than 4 |
1 mL (10 mg) twice daily |
|
4 to less than 5 |
1.5 mL (15 mg) twice daily |
ISENTRESS and ISENTRESS HD can be administered with or without food (2.1).
Do not substitute ISENTRESS chewable tablets or ISENTRESS for oral suspension for the ISENTRESS 400 mg or 600 mg film-coated tablet.
See specific dosing guidance for chewable tablets and the formulation for oral suspension (2.1).
Adults
- Treatment-naïve patients or patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily:
- 1200 mg (2 × 600 mg) film-coated tablet orally, once daily or
- 400 mg film-coated tablet orally, twice daily (2.2).
- Treatment-experienced patients:
- 400 mg film-coated tablet orally, twice daily (2.2).
- During coadministration with rifampin in adults, 800 mg (2 × 400 mg) twice daily (2.2).
Pediatrics
- If weighing at least 40 kg, and either
- treatment-naïve patients or
- patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily:
- 1200 mg (2 × 600 mg) film-coated tablet orally, once daily or
- 400 mg film-coated tablet orally, twice daily or
- 300 mg (3 × 100 mg) chewable tablets, twice daily (2.3).
- If weighing at least 25 kg: One 400 mg film-coated tablet orally, twice daily. If unable to swallow a tablet, consider the chewable tablet, as specified in Table 2 (2.3).
- If weighing at least 3 kg to less than 25 kg: Weight-based dosing using the chewable tablet or oral suspension, as specified in Table 4 (2.3).
- For neonates (birth to 4 weeks [28 days] of age): Weight-based dosing of the oral suspension as specified in Table 5 (2.3).
