INVOKANA
These highlights do not include all the information needed to use INVOKANA safely and effectively. See full prescribing information for INVOKANA. INVOKANA (canagliflozin) tablets, for oral use Initial U.S. Approval: 2013
b9057d3b-b104-4f09-8a61-c61ef9d4a3f3
HUMAN PRESCRIPTION DRUG LABEL
Jul 12, 2023
Janssen Pharmaceuticals, Inc.
DUNS: 063137772
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
canagliflozin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
canagliflozin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label
NDC 50458-141-90
Invokana ®
(canagliflozin) tablets
300 mg
Attention: Dispense the enclosed
Medication Guide to each patient.
Rx only
90 tablets
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and
Other Ketoacidosis
In patients with type 1 diabetes mellitus, INVOKANA significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond the background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium glucose transporter 2 (SGLT2) inhibitors compared to patients who received placebo; this risk may be greater with higher doses of INVOKANA. INVOKANA is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors, including INVOKANA.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include acute febrile illness, reduced caloric intake, ketogenic diet, surgery, insulin dose reduction, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 3 days after discontinuing INVOKANA [see Clinical Pharmacology (12.2)]; however, there have been postmarketing reports of ketoacidosis and glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue INVOKANA, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting INVOKANA.
Withhold INVOKANA, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume INVOKANA when the patient is clinically stable and has resumed oral intake [see Dosage and Administration (2.5)] .
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue INVOKANA and seek medical attention immediately if signs and symptoms occur.
5.2 Lower Limb Amputation
An increased risk of lower limb amputations associated with INVOKANA use versus placebo was observed in CANVAS (5.9 vs 2.8 events per 1000 patient- years) and CANVAS-R (7.5 vs 4.2 events per 1000 patient-years), two randomized, placebo-controlled trials evaluating patients with type 2 diabetes mellitus who had either established cardiovascular disease or were at risk for cardiovascular disease. The risk of lower limb amputations was observed at both the 100 mg and 300 mg once daily dosage regimens. The amputation data for CANVAS and CANVAS-R are shown in Tables 3 and 4, respectively [see Adverse Reactions (6.1)].
Amputations of the toe and midfoot (99 out of 140 patients with amputations receiving INVOKANA in the two trials) were the most frequent; however, amputations involving the leg, below and above the knee, were also observed (41 out of 140 patients with amputations receiving INVOKANA in the two trials). Some patients had multiple amputations, some involving both lower limbs.
Lower limb infections, gangrene, and diabetic foot ulcers were the most common precipitating medical events leading to the need for an amputation. The risk of amputation was highest in patients with a baseline history of prior amputation, peripheral vascular disease, and neuropathy.
Before initiating INVOKANA, consider factors in the patient history that may predispose to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving INVOKANA for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue INVOKANA if these complications occur.
5.3 Volume Depletion
INVOKANA can cause intravascular volume contraction which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)] . There have been post-marketing reports of acute kidney injury which are likely related to volume depletion, some requiring hospitalizations and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including INVOKANA. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m 2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating INVOKANA in patients with one or more of these characteristics, assess and correct volume status. Monitor for signs and symptoms of volume depletion after initiating therapy.
5.4 Urosepsis and Pyelonephritis
There have been postmarketing reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving INVOKANA. Treatment with INVOKANA increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6)] .
5.5 Hypoglycemia with Concomitant Use with Insulin and Insulin
Secretagogues
Insulin and insulin secretagogues are known to cause hypoglycemia. INVOKANA may increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue [see Adverse Reactions (6.1)] . Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with INVOKANA.
5.6 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including INVOKANA. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with INVOKANA presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue INVOKANA, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.7 Genital Mycotic Infections
INVOKANA increases the risk of genital mycotic infections. Patients with a history of genital mycotic infections and uncircumcised males were more likely to develop genital mycotic infections [see Adverse Reactions (6.1)] . Monitor and treat appropriately.
5.8 Hypersensitivity Reactions
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported with INVOKANA. These reactions generally occurred within hours to days after initiating INVOKANA. If hypersensitivity reactions occur, discontinue use of INVOKANA; treat and monitor until signs and symptoms resolve [see Contraindications (4)and Adverse Reactions (6.1, 6.2)] .
5.9 Bone Fracture
An increased risk of bone fracture, occurring as early as 12 weeks after treatment initiation, was observed in patients using INVOKANA in the CANVAS trial [see Clinical Studies (14.2)] . Consider factors that contribute to fracture risk prior to initiating INVOKANA [see Adverse Reactions (6.1)] .
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis, as indicated. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue INVOKANA if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting ( 5.1)
- Lower Limb Amputation: Consider factors that may increase the risk of amputation before initiating INVOKANA. Monitor patients for infection or ulcers of lower limb and discontinue if these occur ( 5.2)
- Volume Depletion: May result in acute kidney injury. Before initiating INVOKANA, assess and correct volume status in patients with renal impairment, elderly patients, or patients on loop diuretics. Monitor for signs and symptoms during therapy ( 5.3)
- Urosepsis and pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated ( 5.4)
- Hypoglycemia: Consider a lower dose of insulin or the insulin secretagogue to reduce the risk of hypoglycemia when used in combination with INVOKANA ( 5.5)
- Necrotizing fasciitis of the perineum (Fournier's gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment ( 5.6)
- Genital mycotic infections: Monitor and treat if indicated ( 5.7)
- Hypersensitivity reactions: Discontinue INVOKANA and monitor until signs and symptoms resolve ( 5.8)
- Bone fracture: Consider factors that contribute to fracture risk before initiating INVOKANA ( 5.9)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 7: Clinically Significant Drug Interactions with INVOKANA|
UGT Enzyme Inducers | |
|
Clinical Impact: |
UGT enzyme inducers decrease canagliflozin exposure which may reduce the effectiveness of INVOKANA. |
|
Intervention: |
For patients with eGFR 60 mL/min/1.73 m 2or greater, if an inducer of UGTs is
administered with INVOKANA, increase the dosage to 200 mg daily in patients
currently tolerating INVOKANA 100 mg daily. The total daily dosage may be
increased to 300 mg daily in patients currently tolerating INVOKANA 200 mg
daily who require additional glycemic control. |
|
Examples: |
Rifampin, phenytoin, phenobarbital, ritonavir |
|
Insulin or Insulin Secretagogues | |
|
Clinical Impact: |
The risk of hypoglycemia is increased when INVOKANA is used concomitantly with insulin secretagogues (e.g., sulfonylurea) or insulin. |
|
Intervention: |
Concomitant use may require a lower dosage of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. |
|
Digoxin | |
|
Clinical Impact: |
Canagliflozin increases digoxin exposure [see Clinical Pharmacology (12.3)] . |
|
Intervention: |
Monitor patients taking INVOKANA with concomitant digoxin for a need to adjust the dosage of digoxin. |
|
Lithium | |
|
Clinical Impact: |
Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
|
Intervention: |
Monitor serum lithium concentration more frequently during INVOKANA initiation and dosage changes. |
|
Drug/Laboratory Test Interference | |
|
Positive Urine Glucose Test | |
|
Clinical Impact: |
SGLT2 inhibitors increase urinary glucose excretion which will lead to positive urine glucose tests. |
|
Intervention: |
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
|
Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
|
Clinical Impact: |
Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
|
Intervention: |
Monitoring glycemic control with 1,5-AG assay is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
See full prescribing information for information on drug interactions and interference of INVOKANA with laboratory tests. ( 7)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
INVOKANA ®(canagliflozin) tablets are available in the strengths and packages listed below:
100 mg tablets are yellow, capsule-shaped, film-coated tablets with "CFZ" on one side and "100" on the other side.
|
NDC 50458-140-30 |
Bottle of 30 |
|
NDC 50458-140-90 |
Bottle of 90 |
|
NDC 50458-140-50 |
Bottle of 500 |
300 mg tablets are white, capsule-shaped, film-coated tablets with "CFZ" on one side and "300" on the other side.
|
NDC 50458-141-30 |
Bottle of 30 |
|
NDC 50458-141-90 |
Bottle of 90 |
|
NDC 50458-141-50 |
Bottle of 500 |
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 07/2023 | ||||||
|
Medication Guide | |||||||
|
What is the most important information I should know about INVOKANA? INVOKANA can cause serious side effects, including: ***Diabetic ketoacidosis (increased ketones in your blood or urine) in people with type 1 and other ketoacidosis.**INVOKANA can cause ketoacidosis that can be life-threatening and may lead to death. People with type 1 diabetes, type 2 diabetes, or pancreas problems have a high risk of getting ketoacidosis. Ketoacidosis can happen even if your blood sugar is less than 250 mg/dL. Your healthcare provider may ask you to periodically check ketones in your urine or blood. Ketoacidosis can also happen in people who are sick or who have surgery during treatment with INVOKANA. Ketoacidosis is a serious condition which needs to be treated in a hospital. Ketoacidosis may lead to death. *Stop taking INVOKANA and call your healthcare provider or get medical help right away if you get any of the following. If possible, check for ketones in your urine or blood, even if your blood sugar is less than 250 mg/dL: | |||||||
|
| ||||||
|
*Amputations. INVOKANA may increase your risk of lower limb amputations. Amputations mainly involve removal of the toe or part of the foot, however, amputations involving the leg, below and above the knee, have also occurred. Some people had more than one amputation, some on both sides of the body. **Call your doctor right away if you have new pain or tenderness, any sores, ulcers, or infections in your leg or foot.**Your doctor may decide to stop your INVOKANA for a while if you have any of these signs or symptoms. Talk to your doctor about proper foot care. | |||||||
|
*Dehydration. INVOKANA can cause some people to become dehydrated (the loss of too much body water). Dehydration may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension). There have been reports of sudden worsening of kidney function in people with type 2 diabetes who are taking INVOKANA. Talk to your doctor about what you can do to prevent dehydration including how much fluid you should drink on a daily basis. Call your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you cannot eat or you start to lose liquids from your body, for example from vomiting, diarrhea, or being in the sun too long. | |||||||
|
***Vaginal yeast infection.**Symptoms of a vaginal yeast infection include: * vaginal odor * white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese) * vaginal itching ***Yeast infection of the skin around the penis (balanitis or balanoposthitis).**Swelling of an uncircumcised penis may develop that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include: | |||||||
|
| ||||||
|
Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis. Your doctor may suggest you use an over-the-counter antifungal medicine. Talk to your doctor right away if you use an over-the- counter antifungal medication and your symptoms do not go away. | |||||||
|
What is INVOKANA?
| |||||||
|
Do not take INVOKANA if you:
| |||||||
|
Before taking INVOKANA, tell your doctor about all of your medical conditions, including if you:
**Tell your doctor about all the medicines you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements. INVOKANA may affect the way other medicines work, and other medicines may affect how INVOKANA works. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. | |||||||
|
How should I take INVOKANA?
| |||||||
|
What are the possible side effects of INVOKANA? INVOKANA may cause serious side effects including: See "What is the most important information I should know about INVOKANA?" ***serious urinary tract infections.**Serious urinary tract infections that may lead to hospitalization have happened in people who are taking INVOKANA. Tell your doctor if you have any signs or symptoms of a urinary tract infection such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people may also have a fever, back pain, nausea, or vomiting.
***low blood sugar (hypoglycemia).**If you take INVOKANA with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take INVOKANA. | |||||||
|
|
| |||||
|
***a rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum).**Necrotizing fasciitis of the perineum has happened in people who take INVOKANA. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries, and may lead to death.Seek medical attention immediately if you have fever or you are feeling very weak, tired or uncomfortable (malaise) and you develop any of the following symptoms in the area between and around your anus and genitals: | |||||||
|
|
| |||||
|
*serious allergic reaction.If you have any symptoms of a serious allergic reaction, stop taking INVOKANA and call your doctor right away or go to the nearest hospital emergency room. See"Do not take INVOKANA if you:". Your doctor may give you a medicine for your allergic reaction and prescribe a different medicine for your diabetes. ***broken bones (fractures).**Bone fractures have been seen in patients taking INVOKANA. Talk to your doctor about factors that may increase your risk of bone fracture. The most common side effects of INVOKANA include:
These are not all the possible side effects of INVOKANA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Janssen Pharmaceuticals, Inc. at 1-800-526-7736. | |||||||
|
How should I store INVOKANA?
| |||||||
|
General information about the safe and effective use of INVOKANA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INVOKANA for a condition for which it was not prescribed. Do not give INVOKANA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about INVOKANA that is written for health professionals. | |||||||
|
What are the ingredients in INVOKANA? Active ingredient: canagliflozin Inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, magnesium stearate, and microcrystalline cellulose. In addition, the tablet coating contains iron oxide yellow E172 (100 mg tablet only), macrogol/PEG, polyvinyl alcohol, talc, and titanium dioxide. | |||||||
|
Active ingredient made in Belgium. Manufactured for: Janssen Pharmaceuticals,
Inc., Titusville, NJ 08560. Licensed from Mitsubishi Tanabe Pharma
Corporation. For patent information: www.janssenpatents.com © 2013 – 2019
Janssen Pharmaceutical Companies | |||||||
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
INVOKANA (canagliflozin) has been studied as monotherapy, in combination with metformin, sulfonylurea, metformin and sulfonylurea, metformin and sitagliptin, metformin and a thiazolidinedione (i.e., pioglitazone), and in combination with insulin (with or without other anti-hyperglycemic agents). The efficacy of INVOKANA was compared to a dipeptidyl peptidase-4 (DPP-4) inhibitor (sitagliptin), both as add-on combination therapy with metformin and sulfonylurea, and a sulfonylurea (glimepiride), both as add-on combination therapy with metformin. INVOKANA was also evaluated in adults 55 to 80 years of age and patients with moderate renal impairment.
Monotherapy
A total of 584 patients with type 2 diabetes inadequately controlled on diet and exercise participated in a 26-week double-blind, placebo-controlled trial to evaluate the efficacy and safety of INVOKANA. The mean age was 55 years, 44% of patients were men, and the mean baseline eGFR was 87 mL/min/1.73 m 2. Patients taking other antihyperglycemic agents (N=281) discontinued the agent and underwent an 8-week washout followed by a 2-week, single-blind, placebo run-in period. Patients not taking oral antihyperglycemic agents (N=303) entered the 2-week, single-blind, placebo run-in period directly. After the placebo run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily for 26 weeks.
At the end of treatment, INVOKANA 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo. INVOKANA 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reduction in fasting plasma glucose (FPG), in improved postprandial glucose (PPG), and in percent body weight reduction compared to placebo (see Table 10). Statistically significant (p<0.001 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -3.7 mmHg and -5.4 mmHg with INVOKANA 100 mg and 300 mg, respectively.
Table 10: Results from 26-Week Placebo-Controlled Clinical Study with INVOKANA as Monotherapy *|
Efficacy Parameter |
Placebo |
INVOKANA |
INVOKANA |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
7.97 |
8.06 |
8.01 |
|
Change from baseline (adjusted mean) |
0.14 |
-0.77 |
-1.03 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.91 ‡ |
-1.16 ‡ | |
|
Percent of Patients Achieving HbA1C< 7% |
21 |
45 ‡ |
62 ‡ |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline (mean) |
166 |
172 |
173 |
|
Change from baseline (adjusted mean) |
8 |
-27 |
-35 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-36 ‡ |
-43 ‡ | |
|
2-hour Postprandial Glucose (mg/dL) | |||
|
Baseline (mean) |
229 |
250 |
254 |
|
Change from baseline (adjusted mean) |
5 |
-43 |
-59 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-48 ‡ |
-64 ‡ | |
|
Body Weight | |||
|
Baseline (mean) in kg |
87.5 |
85.9 |
86.9 |
|
% change from baseline (adjusted mean) |
-0.6 |
-2.8 |
-3.9 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-2.2 ‡ |
-3.3 ‡ |
Add-on Combination Therapy with Metformin
A total of 1,284 patients with type 2 diabetes inadequately controlled on metformin monotherapy (greater than or equal to 2,000 mg/day, or at least 1,500 mg/day if higher dose not tolerated) participated in a 26-week, double- blind, placebo- and active-controlled trial to evaluate the efficacy and safety of INVOKANA in combination with metformin. The mean age was 55 years, 47% of patients were men, and the mean baseline eGFR was 89 mL/min/1.73 m 2. Patients already on the required metformin dose (N=1009) were randomized after completing a 2-week, single-blind, placebo run-in period. Patients taking less than the required metformin dose or patients on metformin in combination with another antihyperglycemic agent (N=275) were switched to metformin monotherapy (at doses described above) for at least 8 weeks before entering the 2-week, single-blind, placebo run-in. After the placebo run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, sitagliptin 100 mg, or placebo, administered once daily as add-on therapy to metformin.
At the end of treatment, INVOKANA 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin. INVOKANA 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reduction in fasting plasma glucose (FPG), in improved postprandial glucose (PPG), and in percent body weight reduction compared to placebo when added to metformin (see Table 11). Statistically significant (p<0.001 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -5.4 mmHg and -6.6 mmHg with INVOKANA 100 mg and 300 mg, respectively.
Table 11: Results from 26-Week Placebo-Controlled Clinical Study of INVOKANA in Combination with Metformin *|
Efficacy Parameter |
Placebo + Metformin |
INVOKANA 100 mg + Metformin |
INVOKANA 300 mg + Metformin |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
7.96 |
7.94 |
7.95 |
|
Change from baseline (adjusted mean) |
-0.17 |
-0.79 |
-0.94 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.62 ‡ |
-0.77 ‡ | |
|
Percent of patients achieving HbA1C< 7% |
30 |
46 ‡ |
58 ‡ |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline (mean) |
164 |
169 |
173 |
|
Change from baseline (adjusted mean) |
2 |
-27 |
-38 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-30 ‡ |
-40 ‡ | |
|
2-hour Postprandial Glucose (mg/dL) | |||
|
Baseline (mean) |
249 |
258 |
262 |
|
Change from baseline (adjusted mean) |
-10 |
-48 |
-57 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-38 ‡ |
-47 ‡ | |
|
Body Weight | |||
|
Baseline (mean) in kg |
86.7 |
88.7 |
85.4 |
|
% change from baseline (adjusted mean) |
-1.2 |
-3.7 |
-4.2 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-2.5 ‡ |
-2.9 ‡ |
Initial Combination Therapy with Metformin
A total of 1,186 patients with type 2 diabetes inadequately controlled with diet and exercise participated in a 26-week double-blind, active-controlled, parallel-group, 5-arm, multicenter trial to evaluate the efficacy and safety of initial therapy with INVOKANA in combination with metformin XR. The median age was 56 years, 48% of patients were men, and the mean baseline eGFR was 87.6 mL/min/1.73 m 2. The median duration of diabetes was 1.6 years, and 72% of patients were treatment naïve. After completing a 2-week single-blind placebo run-in period, patients were randomly assigned for a double-blind treatment period of 26 weeks to 1 of 5 treatment groups (Table 12). The metformin XR dose was initiated at 500 mg/day for the first week of treatment and then increased to 1000 mg/day. Metformin XR or matching placebo was up- titrated every 2–3 weeks during the next 8 weeks of treatment to a maximum daily dose of 1500 to 2000 mg/day, as tolerated; about 90% of patients reached 2000 mg/day.
At the end of treatment, INVOKANA 100 mg and INVOKANA 300 mg in combination with metformin XR resulted in a statistically significant greater improvement in HbA 1Ccompared to their respective INVOKANA doses (100 mg and 300 mg) alone or metformin XR alone.
Table 12: Results from 26-Week Active-Controlled Clinical Study of INVOKANA Alone or INVOKANA as Initial Combination Therapy with Metformin *|
Efficacy Parameter |
Metformin XR |
INVOKANA 100 mg |
INVOKANA 300 mg |
INVOKANA 100 mg + Metformin XR |
INVOKANA 300 mg + Metformin XR |
|---|---|---|---|---|---|
| |||||
|
HbA1C(%) | |||||
|
Baseline (mean) |
8.81 |
8.78 |
8.77 |
8.83 |
8.90 |
|
Change from baseline (adjusted mean) † |
-1.30 |
-1.37 |
-1.42 |
-1.77 |
-1.78 |
|
Difference from canagliflozin 100 mg (adjusted mean) (95% CI) ‡ |
-0.40 § | ||||
|
Difference from canagliflozin 300 mg (adjusted mean) (95% CI) ‡ |
-0.36 § | ||||
|
Difference from metformin XR (adjusted mean) (95% CI) ‡ |
-0.06 ¶ |
-0.11 ¶ |
-0.46 § |
-0.48 § | |
|
Percent of patients achieving HbA1C< 7% |
38 |
34 |
39 |
47 # |
51 # |
INVOKANA Compared to Glimepiride, Both as Add-on Combination With Metformin
A total of 1,450 patients with type 2 diabetes inadequately controlled on metformin monotherapy (greater than or equal to 2,000 mg/day, or at least 1,500 mg/day if higher dose not tolerated) participated in a 52-week, double- blind, active-controlled trial to evaluate the efficacy and safety of INVOKANA in combination with metformin.
The mean age was 56 years, 52% of patients were men, and the mean baseline eGFR was 90 mL/min/1.73 m 2. Patients tolerating maximally required metformin dose (N=928) were randomized after completing a 2-week, single-blind, placebo run-in period. Other patients (N=522) were switched to metformin monotherapy (at doses described above) for at least 10 weeks, then completed a 2-week single-blind run-in period. After the 2-week run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or glimepiride (titration allowed throughout the 52-week trial to 6 or 8 mg), administered once daily as add-on therapy to metformin.
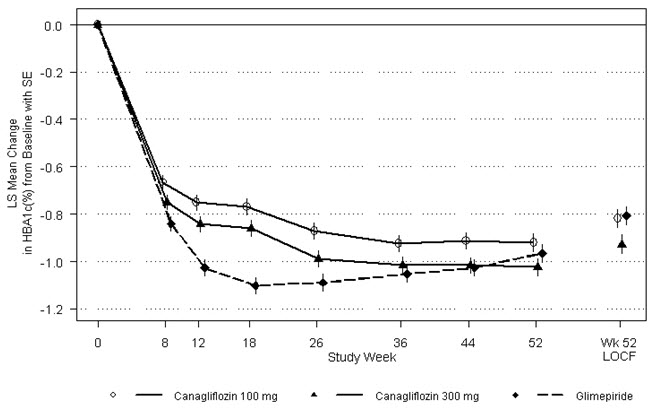
As shown in Table 13 and Figure 1, at the end of treatment, INVOKANA 100 mg provided similar reductions in HbA 1Cfrom baseline compared to glimepiride when added to metformin therapy. INVOKANA 300 mg provided a greater reduction from baseline in HbA 1Ccompared to glimepiride, and the relative treatment difference was -0.12% (95% CI: –0.22; –0.02). As shown in Table 13, treatment with INVOKANA 100 mg and 300 mg daily provided greater improvements in percent body weight change, relative to glimepiride.
Table 13: Results from 52–Week Clinical Study Comparing INVOKANA to Glimepiride in Combination with Metformin *|
Efficacy Parameter |
INVOKANA 100 mg + Metformin |
INVOKANA 300 mg + Metformin |
Glimepiride (titrated) + Metformin |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
7.78 |
7.79 |
7.83 |
|
Change from baseline (adjusted mean) |
-0.82 |
-0.93 |
-0.81 |
|
Difference from glimepiride (adjusted mean) (95% CI) † |
-0.01 ‡ |
-0.12 ‡ | |
|
Percent of patients achieving HbA1C< 7% |
54 |
60 |
56 |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline (mean) |
165 |
164 |
166 |
|
Change from baseline (adjusted mean) |
-24 |
-28 |
-18 |
|
Difference from glimepiride (adjusted mean) (95% CI) † |
-6 |
-9 | |
|
Body Weight | |||
|
Baseline (mean) in kg |
86.8 |
86.6 |
86.6 |
|
% change from baseline (adjusted mean) |
-4.2 |
-4.7 |
1.0 |
|
Difference from glimepiride (adjusted mean) (95% CI) † |
-5.2 § |
-5.7 § |
|
Figure 1: Mean HbA 1CChange at Each Time Point (Completers) and at Week 52 Using Last Observation Carried Forward (mITT Population) |
|---|
|
|
Add-on Combination Therapy with Sulfonylurea
A total of 127 patients with type 2 diabetes inadequately controlled on sulfonylurea monotherapy participated in an 18-week, double-blind, placebo- controlled sub-study to evaluate the efficacy and safety of INVOKANA in combination with sulfonylurea. The mean age was 65 years, 57% of patients were men, and the mean baseline eGFR was 69 mL/min/1.73 m 2. Patients treated with sulfonylurea monotherapy on a stable protocol-specified dose (greater than or equal to 50% maximal dose) for at least 10 weeks completed a 2-week, single- blind, placebo run-in period. After the run-in period, patients with inadequate glycemic control were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily as add-on to sulfonylurea.
As shown in Table 14, at the end of treatment, INVOKANA 100 mg and 300 mg daily provided statistically significant (p<0.001 for both doses) improvements in HbA 1Crelative to placebo when added to sulfonylurea. INVOKANA 300 mg once daily compared to placebo resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, (33% vs 5%), greater reductions in fasting plasma glucose (-36 mg/dL vs +12 mg/dL), and greater percent body weight reduction (-2.0% vs -0.2%).
Table 14: Results from 18-Week Placebo–Controlled Clinical Study of INVOKANA in Combination with Sulfonylurea *|
Efficacy Parameter |
Placebo + Sulfonylurea |
INVOKANA 100 mg + Sulfonylurea |
INVOKANA 300 mg + Sulfonylurea |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
8.49 |
8.29 |
8.28 |
|
Change from baseline (adjusted mean) |
0.04 |
-0.70 |
-0.79 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.74 ‡ |
-0.83 ‡ |
Add-on Combination Therapy with Metformin and Sulfonylurea
A total of 469 patients with type 2 diabetes inadequately controlled on the combination of metformin (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) and sulfonylurea (maximal or near- maximal effective dose) participated in a 26-week, double-blind, placebo- controlled trial to evaluate the efficacy and safety of INVOKANA in combination with metformin and sulfonylurea. The mean age was 57 years, 51% of patients were men, and the mean baseline eGFR was 89 mL/min/1.73 m 2. Patients already on the protocol-specified doses of metformin and sulfonylurea (N=372) entered a 2-week, single-blind, placebo run-in period. Other patients (N=97) were required to be on a stable protocol-specified dose of metformin and sulfonylurea for at least 8 weeks before entering the 2-week run-in period. Following the run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily as add-on to metformin and sulfonylurea.
At the end of treatment, INVOKANA 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin and sulfonylurea. INVOKANA 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in a significant reduction in fasting plasma glucose (FPG), and in percent body weight reduction compared to placebo when added to metformin and sulfonylurea (see Table 15).
Table 15: Results from 26-Week Placebo-Controlled Clinical Study of INVOKANA in Combination with Metformin and Sulfonylurea *|
Efficacy Parameter |
Placebo + Metformin and Sulfonylurea |
INVOKANA 100 mg + Metformin and Sulfonylurea |
INVOKANA 300 mg + Metformin and Sulfonylurea |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
8.12 |
8.13 |
8.13 |
|
Change from baseline (adjusted mean) |
-0.13 |
-0.85 |
-1.06 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.71 ‡ |
-0.92 ‡ | |
|
Percent of patients achieving A1C< 7% |
18 |
43 ‡ |
57 ‡ |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline (mean) |
170 |
173 |
168 |
|
Change from baseline (adjusted mean) |
4 |
-18 |
-31 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-22 ‡ |
-35 ‡ | |
|
Body Weight | |||
|
Baseline (mean) in kg |
90.8 |
93.5 |
93.5 |
|
% change from baseline (adjusted mean) |
-0.7 |
-2.1 |
-2.6 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-1.4 ‡ |
-2.0 ‡ |
Add-on Combination Therapy with Metformin and Sitagliptin
A total of 217 patients with type 2 diabetes inadequately controlled on the combination of metformin (greater than or equal to 1,500 mg/day) and sitagliptin 100 mg/day (or equivalent fixed-dose combination) participated in a 26-week, double-blind, placebo-controlled trial to evaluate the efficacy and safety of INVOKANA in combination with metformin and sitagliptin. The mean age was 57 years, 58% of patients were men, 73% of patients were Caucasian, 15% were Asian, and 12% were Black or African-American. The mean baseline eGFR was 90 mL/min/1.73 m 2and the mean baseline BMI was 32 kg/m 2. The mean duration of diabetes was 10 years. Eligible patients entered a 2-week, single-blind, placebo run-in period and were subsequently randomized to INVOKANA 100 mg or placebo, administered once daily as add-on to metformin and sitagliptin. Patients with a baseline eGFR of 70 mL/min/1.73 m 2or greater who were tolerating INVOKANA 100 mg and who required additional glycemic control (fasting finger stick 100 mg/dL or greater at least twice within 2 weeks) were up-titrated to INVOKANA 300 mg. While up-titration occurred as early as Week 4, most (90%) patients randomized to INVOKANA were up-titrated to INVOKANA 300 mg by 6 to 8 weeks.
At the end of 26 weeks, INVOKANA resulted in a statistically significant improvement in HbA 1C(p<0.001) compared to placebo when added to metformin and sitagliptin.
Table 16: Results from 26–Week Placebo-Controlled Clinical Study of INVOKANA in Combination with Metformin and Sitagliptin|
Efficacy Parameter |
Placebo + Metformin and Sitagliptin |
INVOKANA + Metformin and Sitagliptin |
|---|---|---|
| ||
|
HbA1C(%) | ||
|
Baseline (mean) |
8.40 |
8.50 |
|
Change from baseline (adjusted mean) |
-0.03 |
-0.83 |
|
Difference from placebo (adjusted mean) (95% CI) †‡ |
-0.81 § | |
|
Percent of patients achieving HbA1C< 7%¶ |
9 |
28 |
|
Fasting Plasma Glucose (mg/dL)# | ||
|
Baseline (mean) |
180 |
185 |
|
Change from baseline (adjusted mean) |
-3 |
-28 |
|
Difference from placebo (adjusted mean) (95% CI) |
-25 § |
INVOKANA Compared to Sitagliptin, Both as Add-on Combination Therapy with Metformin and Sulfonylurea
A total of 755 patients with type 2 diabetes inadequately controlled on the combination of metformin (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) and sulfonylurea (near-maximal or maximal effective dose) participated in a 52-week, double-blind, active- controlled trial to compare the efficacy and safety of INVOKANA 300 mg versus sitagliptin 100 mg in combination with metformin and sulfonylurea. The mean age was 57 years, 56% of patients were men, and the mean baseline eGFR was 88 mL/min/1.73 m 2. Patients already on protocol-specified doses of metformin and sulfonylurea (N=716) entered a 2-week single-blind, placebo run-in period. Other patients (N=39) were required to be on a stable protocol-specified dose of metformin and sulfonylurea for at least 8 weeks before entering the 2-week run-in period. Following the run-in period, patients were randomized to INVOKANA 300 mg or sitagliptin 100 mg as add-on to metformin and sulfonylurea.
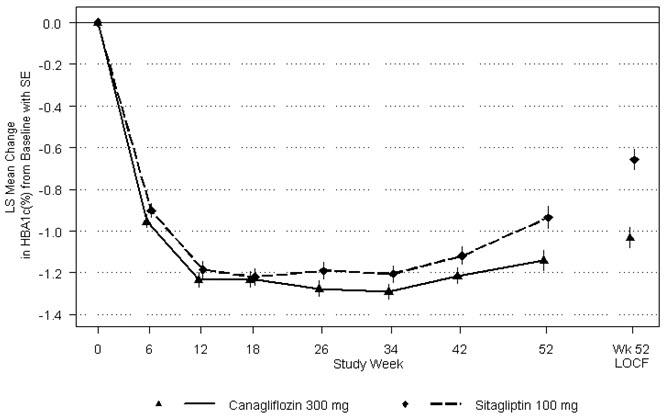
As shown in Table 17 and Figure 2, at the end of treatment, INVOKANA 300 mg provided greater HbA 1Creduction compared to sitagliptin 100 mg when added to metformin and sulfonylurea (p<0.05). INVOKANA 300 mg resulted in a mean percent change in body weight from baseline of -2.5% compared to +0.3% with sitagliptin 100 mg. A mean change in systolic blood pressure from baseline of -5.06 mmHg was observed with INVOKANA 300 mg compared to +0.85 mmHg with sitagliptin 100 mg.
Table 17: Results from 52-Week Clinical Study Comparing INVOKANA to Sitagliptin in Combination with Metformin and Sulfonylurea *|
Efficacy Parameter |
INVOKANA 300 mg + Metformin and Sulfonylurea |
Sitagliptin 100 mg + Metformin and Sulfonylurea |
|---|---|---|
| ||
|
HbA1C(%) | ||
|
Baseline (mean) |
8.12 |
8.13 |
|
Change from baseline (adjusted mean) |
-1.03 |
-0.66 |
|
Difference from sitagliptin (adjusted mean) (95% CI) † |
-0.37 ‡ | |
|
Percent of patients achieving HbA1C< 7% |
48 |
35 |
|
Fasting Plasma Glucose (mg/dL) | ||
|
Baseline (mean) |
170 |
164 |
|
Change from baseline (adjusted mean) |
-30 |
-6 |
|
Difference from sitagliptin (adjusted mean) (95% CI) † |
-24 | |
|
Body Weight | ||
|
Baseline (mean) in kg |
87.6 |
89.6 |
|
% change from baseline (adjusted mean) |
-2.5 |
0.3 |
|
Difference from sitagliptin (adjusted mean) (95% CI) † |
-2.8 § |
Figure 2: Mean HbA1CChange at Each Time Point (Completers) and at Week 52 Using Last Observation Carried Forward (mITT Population)
Add-on Combination Therapy with Metformin and Pioglitazone
A total of 342 patients with type 2 diabetes inadequately controlled on the combination of metformin (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) and pioglitazone (30 or 45 mg/day) participated in a 26-week, double-blind, placebo-controlled trial to evaluate the efficacy and safety of INVOKANA in combination with metformin and pioglitazone. The mean age was 57 years, 63% of patients were men, and the mean baseline eGFR was 86 mL/min/1.73 m 2. Patients already on protocol- specified doses of metformin and pioglitazone (N=163) entered a 2-week, single-blind, placebo run-in period. Other patients (N=181) were required to be on stable protocol-specified doses of metformin and pioglitazone for at least 8 weeks before entering the 2-week run-in period. Following the run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily as add-on to metformin and pioglitazone.
At the end of treatment, INVOKANA 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin and pioglitazone. INVOKANA 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reduction in fasting plasma glucose (FPG) and in percent body weight reduction compared to placebo when added to metformin and pioglitazone (see Table 18). Statistically significant (p<0.05 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -4.1 mmHg and -3.5 mmHg with INVOKANA 100 mg and 300 mg, respectively.
Table 18: Results from 26-Week Placebo-Controlled Clinical Study of INVOKANA in Combination with Metformin and Pioglitazone *|
Efficacy Parameter |
Placebo + Metformin and Pioglitazone |
INVOKANA 100 mg + Metformin and Pioglitazone |
INVOKANA 300 mg + Metformin and Pioglitazone |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
8.00 |
7.99 |
7.84 |
|
Change from baseline (adjusted mean) |
-0.26 |
-0.89 |
-1.03 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.62 ‡ |
-0.76 ‡ | |
|
Percent of patients achieving HbA1C< 7% |
33 |
47 ‡ |
64 ‡ |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline (mean) |
164 |
169 |
164 |
|
Change from baseline (adjusted mean) |
3 |
-27 |
-33 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-29 ‡ |
-36 ‡ | |
|
Body Weight | |||
|
Baseline (mean) in kg |
94.0 |
94.2 |
94.4 |
|
% change from baseline (adjusted mean) |
-0.1 |
-2.8 |
-3.8 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-2.7 ‡ |
-3.7 ‡ |
Add-On Combination Therapy with Insulin (With or Without Other Antihyperglycemic Agents)
A total of 1,718 patients with type 2 diabetes inadequately controlled on insulin greater than or equal to 30 units/day or insulin in combination with other antihyperglycemic agents participated in an 18-week, double-blind, placebo-controlled substudy of a cardiovascular trial to evaluate the efficacy and safety of INVOKANA in combination with insulin. The mean age was 63 years, 66% of patients were men, and the mean baseline eGFR was 75 mL/min/1.73 m 2. Patients on basal, bolus, or basal/bolus insulin for at least 10 weeks entered a 2-week, single-blind, placebo run-in period. Approximately 70% of patients were on a background basal/bolus insulin regimen. After the run-in period, patients were randomized to INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily as add-on to insulin. The mean daily insulin dose at baseline was 83 units, which was similar across treatment groups.
At the end of treatment, INVOKANA 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to insulin. INVOKANA 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reductions in fasting plasma glucose (FPG), and in percent body weight reductions compared to placebo (see Table 19). Statistically significant (p<0.001 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -2.6 mmHg and -4.4 mmHg with INVOKANA 100 mg and 300 mg, respectively.
Table 19: Results from 18-Week Placebo-Controlled Clinical Study of INVOKANA in Combination with Insulin ≥ 30 Units/Day (With or Without Other Oral Antihyperglycemic Agents) *|
Efficacy Parameter |
Placebo + Insulin |
INVOKANA 100 mg + Insulin |
INVOKANA 300 mg + Insulin |
|---|---|---|---|
| |||
|
HbA1C(%) | |||
|
Baseline (mean) |
8.20 |
8.33 |
8.27 |
|
Change from baseline (adjusted mean) |
0.01 |
-0.63 |
-0.72 |
|
Difference from placebo (adjusted mean) (95% CI) † |
-0.65 ‡ |
-0.73 ‡ | |
|
Percent of patients achieving HbA1C< 7% |
8 |
20 ‡ |
25 ‡ |
|
Fasting Plasma Glucose (mg/dL) | |||
|
Baseline |
169 |
170 |
168 |
|
Change from baseline (adjusted mean) |
4 |
-19 |
-25 |
|
Difference from placebo (adjusted mean) (97.5% CI) † |
-23 ‡ |
-29 ‡ | |
|
Body Weight | |||
|
Baseline (mean) in kg |
97.7 |
96.9 |
96.7 |
|
% change from baseline (adjusted mean) |
0.1 |
-1.8 |
-2.3 |
|
Difference from placebo (adjusted mean) (97.5% CI) † |
-1.9 ‡ |
-2.4 ‡ |
Study in Patients Ages 55 to 80
A total of 714 type 2 diabetes patients ages 55 to 80 years and inadequately controlled on current diabetes therapy (either diet and exercise alone or in combination with oral or parenteral agents) participated in a 26-week, double- blind, placebo-controlled trial to evaluate the efficacy and safety of INVOKANA in combination with current diabetes treatment. The mean age was 64 years, 55% of patients were men, and the mean baseline eGFR was 77 mL/min/1.73 m 2. Patients were randomized in a 1:1:1 ratio to the addition of INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily. At the end of treatment, INVOKANA provided statistically significant improvements from baseline relative to placebo in HbA 1C(p<0.001 for both doses) of -0.57% (95% CI: -0.71%; -0.44%) for INVOKANA 100 mg and -0.70% (95% CI: -0.84%; -0.57%) for INVOKANA 300 mg [see Use in Specific Populations (8.5)] .
Glycemic Control in Patients with Moderate Renal Impairment
A total of 269 patients with type 2 diabetes and a baseline eGFR of 30 mL/min/1.73 m 2to less than 50 mL/min/1.73 m 2inadequately controlled on current diabetes therapy participated in a 26-week, double-blind, placebo- controlled clinical trial to evaluate the efficacy and safety of INVOKANA in combination with current diabetes treatment (diet or antihyperglycemic agent therapy, with 95% of patients on insulin and/or sulfonylurea). The mean age was 68 years, 61% of patients were men, and the mean baseline eGFR was 39 mL/min/1.73 m 2. Patients were randomized in a 1:1:1 ratio to the addition of INVOKANA 100 mg, INVOKANA 300 mg, or placebo, administered once daily.
At the end of treatment, INVOKANA 100 mg and INVOKANA 300 mg daily provided greater reductions in HbA 1Crelative to placebo (-0.30% [95% CI: -0.53%; -0.07%] and -0.40%, [95% CI: -0.64%; -0.17%], respectively) [see Warnings and Precautions (5.3), Adverse Reactions (6.1), Use in Specific Populations (8.6), and Clinical Studies (14.3)] .
14.2 Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and
Atherosclerotic Cardiovascular Disease
The CANVAS and CANVAS-R trials were multicenter, multi-national, randomized, double-blind parallel group, with similar inclusion and exclusion criteria. Patients eligible for enrollment in both CANVAS and CANVAS-R trials were: 30 years of age or older and had established, stable, cardiovascular, cerebrovascular, peripheral artery disease (66% of the enrolled population) or were 50 years of age or older and had two or more other specified risk factors for cardiovascular disease (34% of the enrolled population).
The integrated analysis of the CANVAS and CANVAS-R trials compared the risk of Major Adverse Cardiovascular Event (MACE) between canagliflozin and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and atherosclerotic cardiovascular disease. The primary endpoint, MACE, was the time to first occurrence of a three-part composite outcome which included cardiovascular death, non-fatal myocardial infarction and non-fatal stroke.
In CANVAS, patients were randomly assigned 1:1:1 to canagliflozin 100 mg, canagliflozin 300 mg, or matching placebo. In CANVAS-R, patients were randomly assigned 1:1 to canagliflozin 100 mg or matching placebo, and titration to 300 mg was permitted at the investigator's discretion (based on tolerability and glycemic needs) after Week 13. Concomitant antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
A total of 10,134 patients were treated (4,327 in CANVAS and 5,807 in CANVAS-R; total of 4,344 randomly assigned to placebo and 5,790 to canagliflozin) for a mean exposure duration of 149 weeks (223 weeks [4.3 years] in CANVAS and 94 weeks [1.8 years] in CANVAS-R) .Approximately 78% of the trial population was Caucasian, 13% was Asian, and 3% was Black. The mean age was 63 years and approximately 64% were male.
The mean HbA 1Cat baseline was 8.2% and mean duration of diabetes was 13.5 years with 70% of patients having had diabetes for 10 years or more. Approximately 31%, 21% and 17% reported a past history of neuropathy, retinopathy and nephropathy, respectively, and the mean eGFR 76 mL/min/1.73 m 2. At baseline, patients were treated with one (19%) or more (80%) antidiabetic medications including metformin (77%), insulin (50%), and sulfonylurea (43%).
At baseline, the mean systolic blood pressure was 137 mmHg, the mean diastolic blood pressure was 78 mmHg, the mean LDL was 89 mg/dL, the mean HDL was 46 mg/dL, and the mean urinary albumin to creatinine ratio (UACR) was 115 mg/g. At baseline, approximately 80% of patients were treated with renin angiotensin system inhibitors, 53% with beta-blockers, 13% with loop diuretics, 36% with non-loop diuretics, 75% with statins, and 74% with antiplatelet agents (mostly aspirin). During the trial, investigators could modify anti-diabetic and cardiovascular therapies to achieve local standard of care treatment targets with respect to blood glucose, lipid, and blood pressure. More patients receiving canagliflozin compared to placebo initiated anti-thrombotics (5.2% vs 4.2%) and statins (5.8% vs 4.8%) during the trial.
For the primary analysis, a stratified Cox proportional hazards model was used to test for non-inferiority against a pre-specified risk margin of 1.3 for the hazard ratio of MACE.
In the integrated analysis of CANVAS and CANVAS-R trials, canagliflozin reduced the risk of first occurrence of MACE. The estimated hazard ratio (95% CI) for time to first MACE was 0.86 (0.75, 0.97). Refer to Table 20. Vital status was obtained for 99.6% of patients across the trials. The Kaplan-Meier curve depicting time to first occurrence of MACE is shown in Figure 3.
Table 20: Treatment Effect for the Primary Composite Endpoint, MACE, and its Components in the Integrated Analysis of CANVAS and CANVAS-R studies *|
Placebo |
Canagliflozin |
Hazard ratio | |
|---|---|---|---|
| |||
|
Composite of cardiovascular death, non-fatal myocardial infarction, non-fatal
stroke |
426 (10.4) |
585 (9.2) |
0.86 (0.75, 0.97) |
|
Non-fatal myocardial infarction §,¶ |
159 (3.9) |
215 (3.4) |
0.85 (0.69, 1.05) |
|
Non-fatal Stroke §,¶ |
116 (2.8) |
158 (2.5) |
0.90 (0.71, 1.15) |
|
Cardiovascular Death §,¶ |
185 (4.6) |
268 (4.1) |
0.87 (0.72, 1.06) |
Figure 3: Time to First Occurrence of MACE
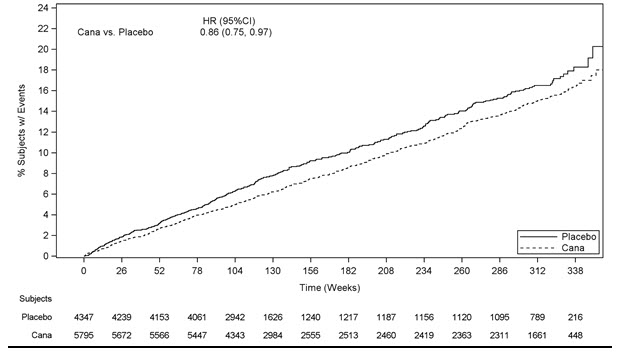
14.3 Renal and Cardiovascular Outcomes in Patients with Diabetic
Nephropathy and Albuminuria
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial (CREDENCE) was a multinational, randomized, double- blind, placebo-controlled trial comparing canagliflozin with placebo in patients with type 2 diabetes mellitus, an eGFR ≥ 30 to < 90 mL/min/1.73 m 2and albuminuria (urine albumin/creatinine > 300 to ≤ 5000 mg/g) who were receiving standard of care including a maximum-tolerated, labeled daily dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB).
The primary objective of CREDENCE was to assess the efficacy of canagliflozin relative to placebo in reducing the composite endpoint of end stage kidney disease (ESKD), doubling of serum creatinine, and renal or CV death.
Patients were randomized to receive canagliflozin 100 mg (N=2,202) or placebo (N=2,199) and treatment was continued until the initiation of dialysis or renal transplantation.
The median follow-up duration for the 4,401 randomized subjects was 137 weeks. Vital status was obtained for 99.9% of subjects.
The population was 67% White, 20% Asian, and 5% Black; 32% were of Hispanic or Latino ethnicity. The mean age was 63 years and 66% were male.
At randomization, the mean HbA 1cwas 8.3%, the median urine albumin/creatinine was 927 mg/g, the mean eGFR was 56.2 mL/min/1.73 m 2, 50% had prior CV disease, and 15% reported a history of heart failure. The most frequent antihyperglycemic agents (AHA) medications used at baseline were insulin (66%), biguanides (58%), and sulfonylureas (29%). Nearly all subjects (99.9%) were on ACEi or ARB at randomization, approximately 60% were taking an anti- thrombotic agent (including aspirin), and 69% were on a statin.
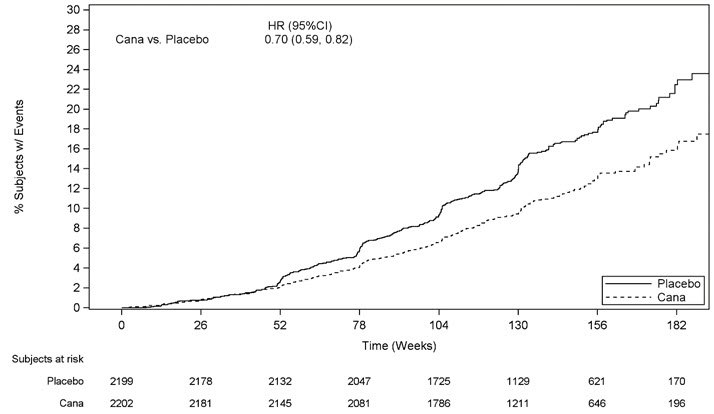
The primary composite endpoint in the CREDENCE study was the time to first occurrence of ESKD (defined as an eGFR < 15 mL/min/1.73 m 2, initiation of chronic dialysis or renal transplant), doubling of serum creatinine, and renal or CV death. Canagliflozin 100 mg significantly reduced the risk of the primary composite endpoint based on a time-to-event analysis [HR: 0.70; 95% CI: 0.59, 0.82; p<0.0001] (see Figure 4). The treatment effect reflected a reduction in progression to ESKD, doubling of serum creatinine and cardiovascular death as shown in Table 21 and Figure 4. There were few renal deaths during the trial. Canagliflozin 100 mg also significantly reduced the risk of hospitalization for heart failure [HR: 0.61; 95% CI: 0.47 to 0.80; p<0.001].
Table 21: Analysis of Primary Endpoint (including the Individual Components) and Secondary Endpoints from the CREDENCE Study|
Placebo |
canagliflozin | ||||
|---|---|---|---|---|---|
|
Endpoint |
N=2,199 (%) |
Event Rate * |
N=2,202 (%) |
Event Rate * |
HR † |
|
Intent-To-Treat Analysis Set (time to first occurrence) | |||||
|
The individual components do not represent a breakdown of the composite outcomes, but rather the total number of subjects experiencing an event during the course of the study. | |||||
| |||||
|
Primary Composite Endpoint (ESKD, doubling of serum creatinine, renal death, or CV death) |
340 (15.5) |
6.1 |
245 (11.1) |
4.3 |
0.70 |
|
ESKD |
165 (7.5) |
2.9 |
116 (5.3) |
2.0 |
0.68 |
|
Doubling of serum creatinine |
188 (8.5) |
3.4 |
118 (5.4) |
2.1 |
0.60 |
|
Renal death |
5 (0.2) |
0.1 |
2 (0.1) |
0.0 | |
|
CV death |
140 (6.4) |
2.4 |
110 (5.0) |
1.9 |
0.78 |
|
CV death or hospitalization for heart failure |
253 (11.5) |
4.5 |
179 (8.1) |
3.1 |
0.69 |
|
CV death, non-fatal myocardial infarction or non-fatal stroke |
269 (12.2) |
4.9 |
217 (9.9) |
3.9 |
0.80 |
|
Non-fatal myocardial infarction |
87 (4.0) |
1.6 |
71 (3.2) |
1.3 |
0.81 |
|
Non-fatal stroke |
66 (3.0) |
1.2 |
53 (2.4) |
0.9 |
0.80 |
|
Hospitalization for heart failure |
141 (6.4) |
2.5 |
89 (4.0) |
1.6 |
0.61 |
|
ESKD, doubling of serum creatinine or renal death |
224 (10.2) |
4.0 |
153 (6.9) |
2.7 |
0.66 |
The Kaplan-Meier curve (Figure 4) shows time to first occurrence of the primary composite endpoint of ESKD, doubling of serum creatinine, renal death, or CV death. The curves begin to separate by Week 52 and continue to diverge thereafter.
Figure 4: CREDENCE: Time to First Occurrence of the Primary Composite Endpoint