CharmTex Lightly Scented Antibacterial
85420-004 CharmTex Antibacterial Soap
1c9f4bff-3f6f-4498-b8a2-7d7f49d6ad2d
HUMAN OTC DRUG LABEL
Jun 1, 2025
Liufangjing Biotechnology (Yangzhou) Co., Ltd
DUNS: 510194010
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
BENZETHONIUM CHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
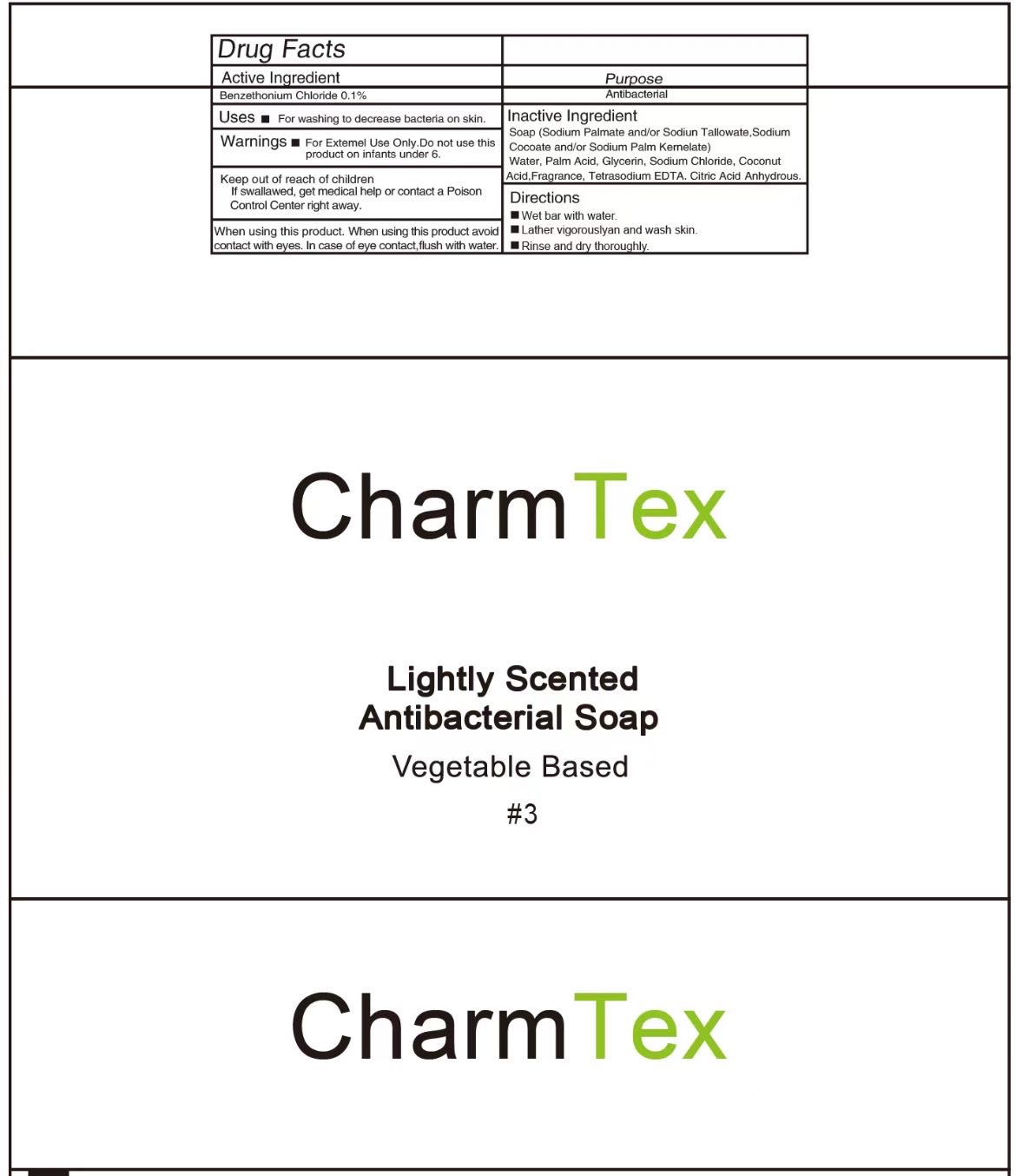
INDICATIONS & USAGE SECTION
Use
For washing to decrease bacteria on skin
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Benzethonium Chloride 0.1%
OTC - PURPOSE SECTION
Purpose
Antibacterial
WARNINGS SECTION
Warnings
For external use only
Do not use this product on infants under 6
OTC - WHEN USING SECTION
When using this product avoid contact with eyes. In case of eye contact flush with water
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Wet bar with water
Lather vigorously and wash skin
Rinse and dry thoroughly
INACTIVE INGREDIENT SECTION
Inactive ingredients
Soap (Sodium Palmate and/or Sodium Tallowate, Sodium Cocoate and/or Sodium Palm Kernelate), Water, Palm Acid, Glycerin, Coconut Acid, Fragrance, tetrasodium EDTA, Citric Acid Anhydrous
