Fomepizole
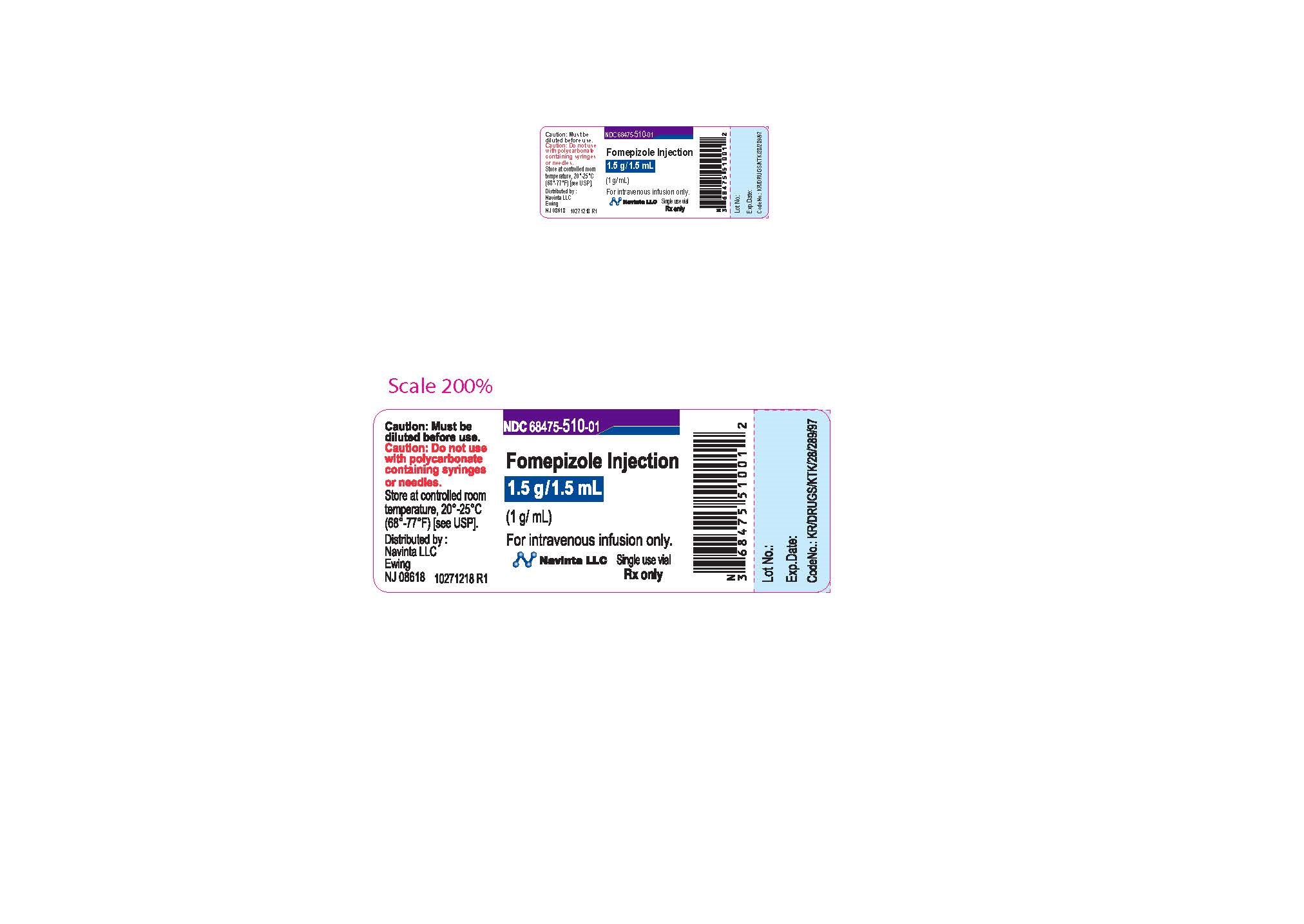
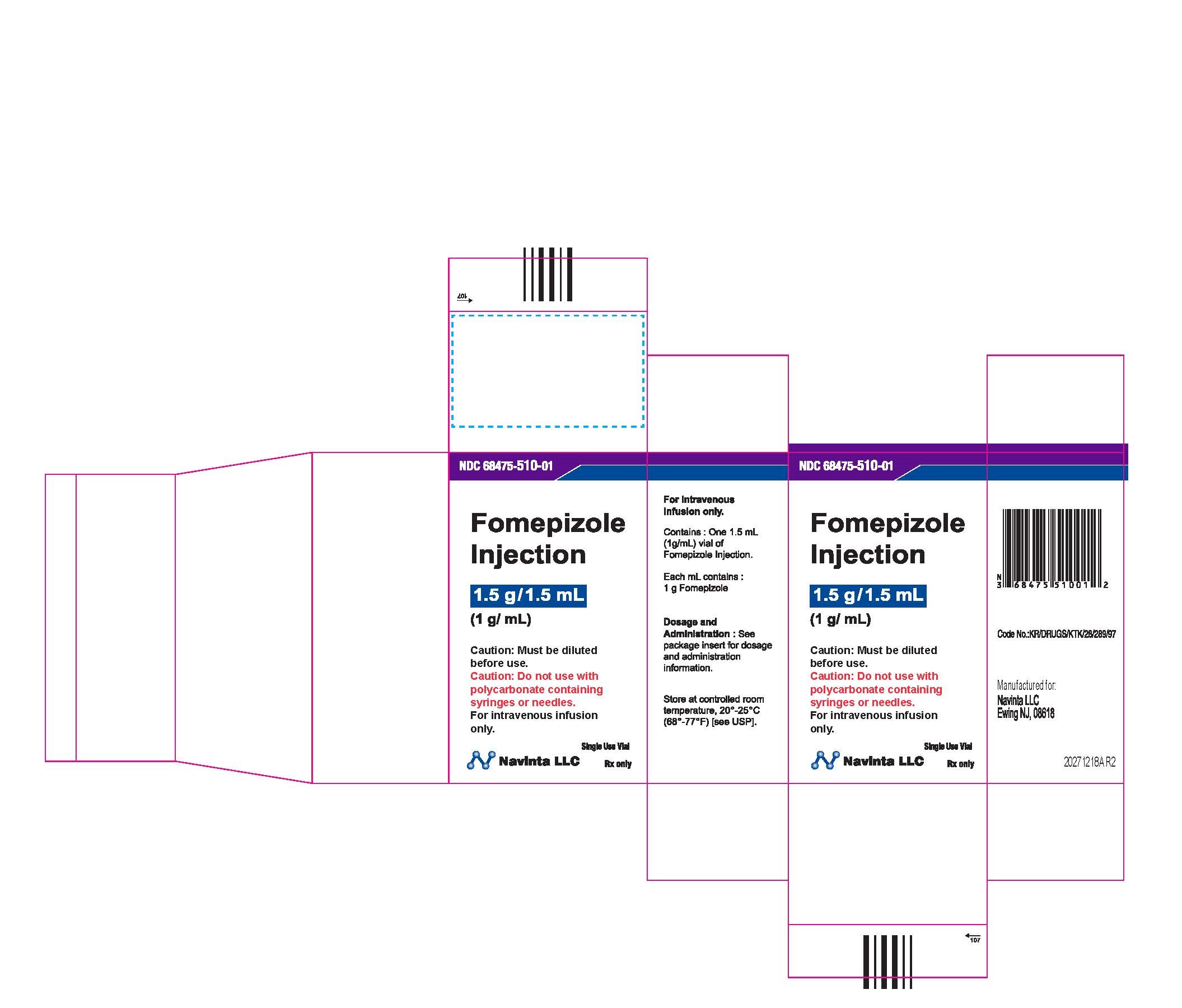
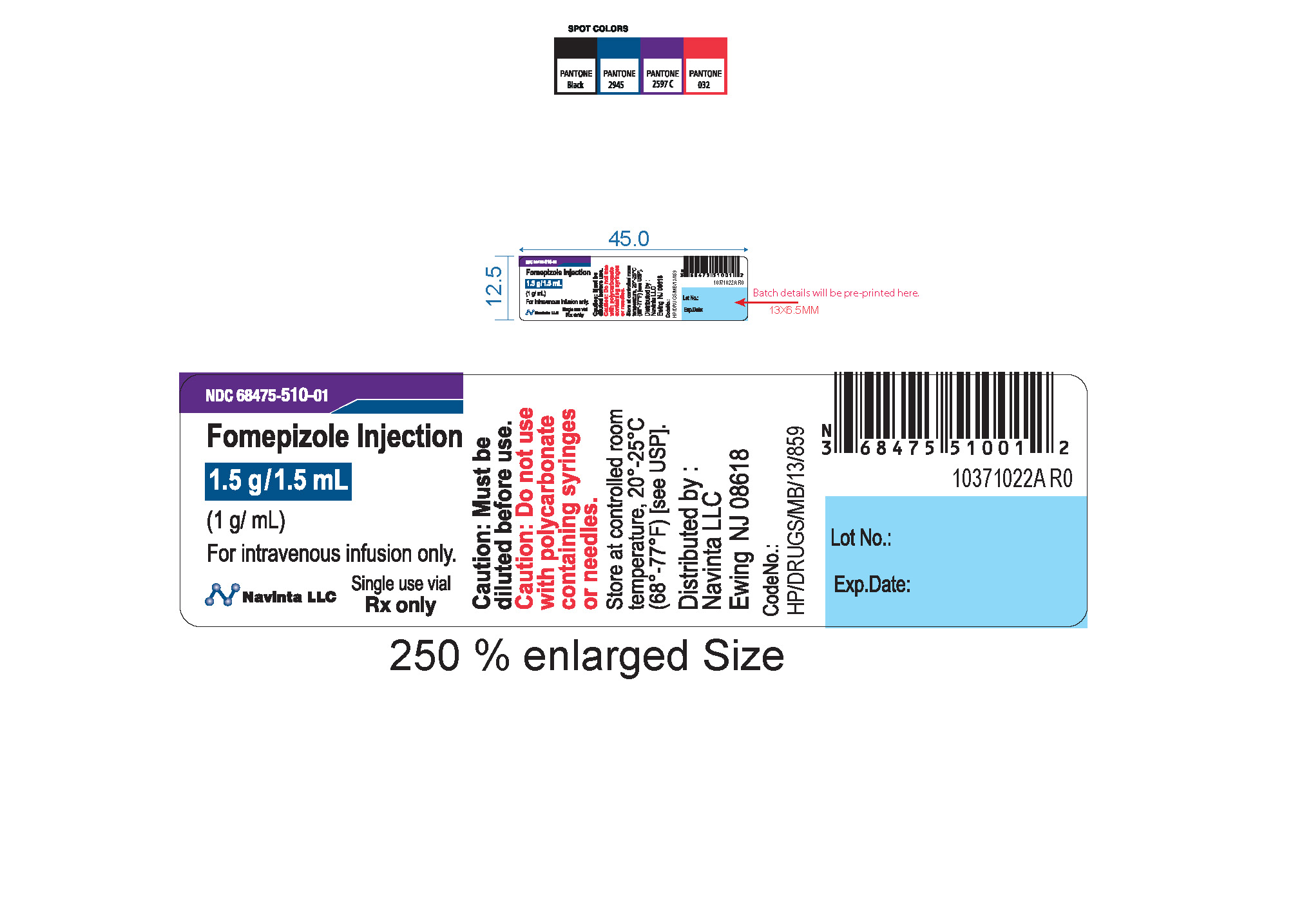
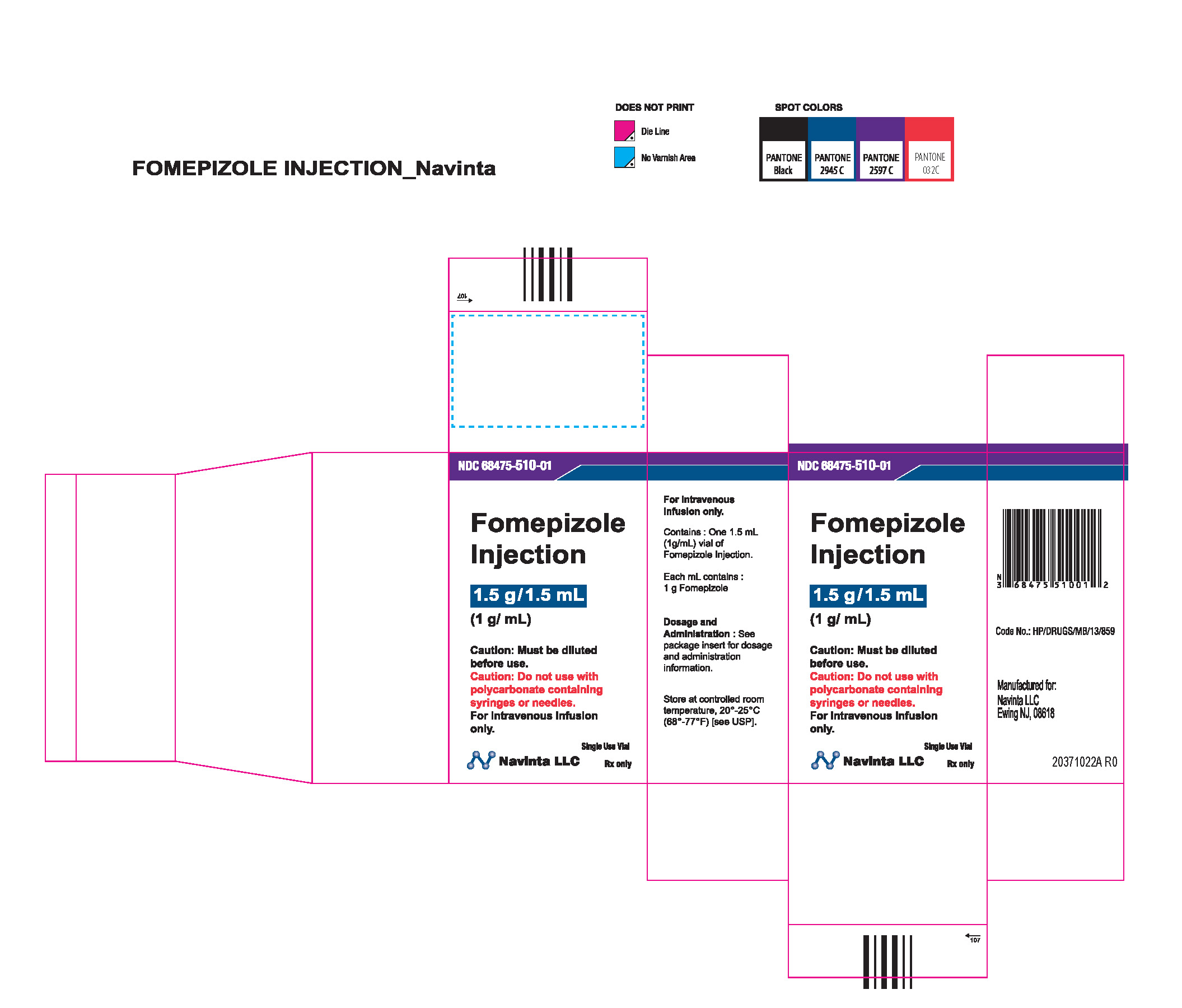
Rx only Sterile Caution : Must be diluted prior to use. Do not use polycarbonate syringes or polycarbonate-containing needles (including polycarbonate filter needles) with fomepizole injection.
Approved
Approval ID
6fed6052-f159-46d6-8be1-31e18a7ba918
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Jan 31, 2023
Manufacturers
FDA
Navinta LLC
DUNS: 130443810
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fomepizole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code68475-510
Application NumberANDA078537
Product Classification
M
Marketing Category
C73584
G
Generic Name
Fomepizole
Product Specifications
Route of AdministrationINTRAVENOUS
Effective DateJanuary 31, 2023
FDA Product Classification
INGREDIENTS (1)
FOMEPIZOLEActive
Quantity: 1 g in 1 mL
Code: 83LCM6L2BY
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 12/9/2022
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL