Lisinopril and Hydrochlorothiazide
Lisinopril and Hydrochlorothiazide Tablets, USP, for oral use Rx Only
62e8c093-cd8e-4342-aadb-209c7e6699c8
HUMAN PRESCRIPTION DRUG LABEL
Mar 12, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lisinopril and Hydrochlorothiazide Tablets
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Lisinopril and hydrochlorothiazide tablets combine an angiotensin converting enzyme inhibitor, lisinopril, and a diuretic, hydrochlorothiazide.
Lisinopril, a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. It is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5 . 2H2O and its structural formula is:
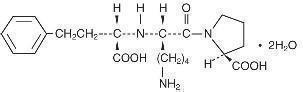
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.52. It is soluble in water, sparingly soluble in methanol, and practically insoluble in ethanol.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
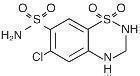
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.73, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Lisinopril and hydrochlorothiazide tablets, USP, are available for oral use in three tablet combinations of lisinopril with hydrochlorothiazide: lisinopril and hydrochlorothiazide tablets 10-12.5 containing 10 mg lisinopril and 12.5 mg hydrochlorothiazide; lisinopril and hydrochlorothiazide tablets 20-12.5 containing 20 mg lisinopril and 12.5 mg hydrochlorothiazide; and, lisinopril and hydrochlorothiazide tablets 20-25 containing 20 mg lisinopril and 25 mg hydrochlorothiazide.
Inactive Ingredients:
10-12.5 Tablets – mannitol, dibasic calcium phosphate dihydrate, pregelatinized starch, magnesium stearate.
20-12.5 Tablets - mannitol, dibasic calcium phosphate dihydrate, pregelatinized starch, magnesium stearate, yellow ferric oxide.
20-25 Tablets - mannitol, dibasic calcium phosphate dihydrate, pregelatinized starch, magnesium stearate, red ferric oxide.
PRECAUTIONS SECTION
PRECAUTIONS
General
Lisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy: As with all vasodilators, lisinopril should be given with caution to patients with obstruction in the outflow tract of the left ventricle.
Impaired Renal Function: As a consequence of inhibiting the renin- angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin- aldosterone system, treatment with angiotensin converting enzyme inhibitors, including lisinopril, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death.
In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur. Experience with another angiotensin-converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of lisinopril and/or diuretic therapy. In such patients renal function should be monitored during the first few weeks of therapy.
Some hypertensive patients with no apparent pre-existing renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when lisinopril has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction of lisinopril and/or discontinuation of the diuretic may be required.
Evaluation of the hypertensive patient should always include assessment of renal function. (SeeDOSAGE AND ADMINISTRATION.****)
Hyperkalemia: In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in approximately 1.4 percent of hypertensive patients treated with lisinopril plus hydrochlorothiazide. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was not a cause of discontinuation of therapy. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes. Hyperkalemia can cause serious, sometimes fatal, arrhythmias. Lisinopril and hydrochlorothiazide tablets should be used cautiously, if at all, with these agents and with frequent monitoring of serum potassium. (See PRECAUTIONS, Drug Interactions.)
Cough: Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor- induced cough should be considered in the differential diagnosis of cough.
Surgery/Anesthesia: In patients undergoing major surgery or during anesthesia with agents that produce hypotension, lisinopril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Hydrochlorothiazide
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present, or after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability). Because lisinopril reduces the production of aldosterone, concomitant therapy with lisinopril attenuates the diuretic-induced potassium loss (see PRECAUTIONS, Drug Interactions, Agents Increasing Serum Potassium).
Although any chloride deficit is generally mild and usually does not require specific treatment, except under extraordinary circumstances (as in liver disease or renal disease), chloride replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required. Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
If progressive renal impairment becomes evident consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Information for Patients
Angioedema: Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin converting enzyme inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Symptomatic Hypotension: Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual syncope occurs, the patients should be told to discontinue the drug until they have consulted with the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with their physician.
Hyperkalemia: Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Neutropenia: Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of neutropenia.
Pregnancy: Female patients of childbearing age should be told about the consequences of exposure to lisinopril and hydrochlorothiazide tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
**Non-melanoma Skin Cancer:**Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Drug Interactions
Lisinopril
Hypotension - Patients on Diuretic Therapy: Patients on diuretics and especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose for at least two hours and until blood pressure has stabilized for at least an additional hour. (See WARNINGS, and DOSAGE AND ADMINISTRATION.) When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed (See DOSAGE AND ADMINISTRATION.)
Non-steroidal Anti-inflammatory Agents Including Selective Cyclooxygenase-2 (COX-2) Inhibitors: In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co- administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including lisinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving lisinopril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including lisinopril, may be attenuated by NSAIDs.
Dual Blockade of the Renin-Angiotensin System(RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or direct renin inhibitors (such as aliskiren) is associated with increased risk of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The VA NEPHRON trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin-to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 ml/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end state renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
In general, avoid combined use of RAS inhibitors. Monitor blood pressure, renal function, and electrolytes in patients on lisinopril and hydrochlorothiazide tablets and other agents that affect the RAS.
Do not coadminister aliskiren with lisinopril and hydrochlorothiazide tablets in patients with diabetes. Avoid use of aliskiren with PRINZIDE in patients with renal impairment (GFR <60 ml/min).
Other Agents: Lisinopril has been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. No meaningful clinically important pharmacokinetic interactions occurred when lisinopril was used concomitantly with propranolol, digoxin, or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium: Lisinopril attenuates potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, eplerenone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated, because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium.
Lithium: Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril is administered concomitantly with lithium.
**Gold:**Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including lisinopril and hydrochlorothiazide tablets.
**mTOR (mammalian target of rapamycin) inhibitors:**Patients receiving coadministration of ACE inhibitor and mTOR inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema. (see WARNINGS)
**Neprilysin Inhibitors:**Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema. (see WARNINGS)
Hydrochlorothiazide
When administered concurrently the following drugs may interact with thiazide diuretics.
Alcohol, barbiturates, or narcotics** -** potentiation of orthostatic hypotension may occur.
Antidiabetic drugs** (oral agents and insulin) -** dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs** -** additive effect or potentiation.
Cholestyramine and colestipol resins** -** Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively.
Corticosteroids, ACTH** -** intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine)** -** possible decreased response to pressor amines but not sufficient to preclude their use.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine)** -** possible increased responsiveness to the muscle relaxant.
Lithium**-** should not generally be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with lisinopril and hydrochlorothiazide tablets.
Non-steroidal Anti-inflammatoryDrugs** -** In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when lisinopril and hydrochlorothiazide tablets and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of lisinopril and hydrochlorothiazide tablets is obtained.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lisinopril-Hydrochlorothiazide
Lisinopril in combination with hydrochlorothiazide was not mutagenic in a microbial mutagen test using Salmonella typhimurium (Ames test) or Escherichia coli with or without metabolic activation or in a forward mutation assay using Chinese hamster lung cells. Lisinopril-hydrochlorothiazide did not produce DNA single strand breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, it did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
Lisinopril
There was no evidence of a tumorigenic effect when lisinopril was administered orally for 105 weeks to male and female rats at doses up to 90 mg/kg/day or for 92 weeks to male and female mice at doses up to 135 mg/kg/day. These doses are 10 times and 7 times, respectively, the maximum recommended human daily dose (MRHDD) when compared on a body surface area basis.
Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
There were no adverse effects on reproductive performance in male and female rats treated with up to 300 mg/kg/day of lisinopril (33 times the MRHDD when compared on a body surface area basis).
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice at doses of up to approximately 600 mg/kg/day (53 times the MRHDD when compared on a body surface area basis) or in male and female rats at doses of up to approximately 100 mg/kg/day (18 times the MRHDD when compared on a body surface area basis). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 mcg/mL to 1300 mcg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation. In mice and rats these doses are 9 times and 0.7 times, respectively, the MRHDD when compared on a body surface area basis.
Nursing Mothers
It is not known whether lisinopril is excreted in human milk. However, milk of lactating rats contains radioactivity following administration of 14C lisinopril. In another study, lisinopril was present in rat milk at levels similar to plasma levels in the dams. Thiazides do appear in human milk. Because of the potential for serious reactions in nursing infants from ACE inhibitors and hydrochlorothiazide, a decision should be made whether to discontinue nursing or to discontinue lisinopril and hydrochlorothiazide tablets, taking into account the importance of the drug to the mother.
Pediatric Use
Neonates with a history of in utero exposure to lisinopril and hydrochlorothiazide tablets:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Lisinopril, which crosses the placenta, has been removed from neonatal circulation by peritoneal dialysis with some clinical benefit, and theoretically may be removed by exchange transfusion, although there is no experience with the latter procedure.
Geriatric Use
Clinical studies of lisinopril and hydrochlorothiazide tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In a multiple-dose pharmacokinetic study in elderly versus young hypertensive patients using the lisinopril/hydrochlorothiazide combination, area under the plasma concentration time curve (AUC) increased approximately 120% for lisinopril and approximately 80% for hydrochlorothiazide in older patients.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Evaluation of the hypertensive patient should always include assessment of renal function. (See DOSAGE AND ADMINISTRATION.)
OVERDOSAGE SECTION
OVERDOSAGE
No specific information is available on the treatment of overdosage with lisinopril and hydrochlorothiazide tablets. Treatment is symptomatic and supportive. Therapy with lisinopril and hydrochlorothiazide tablets should be discontinued and the patient observed closely. Suggested measures include induction of emesis and/or gastric lavage, and correction of dehydration, electrolyte imbalance and hypotension by established procedures.
Lisinopril
Following a single oral dose of 20 g/kg no lethality occurred in rats and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis (see WARNINGS, Anaphylactoid reaction during membrane exposure).
Hydrochlorothiazide
Oral administration of a single oral dose of 10 g/kg to mice and rats was not lethal. The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
