Olopatadine hydrochloride
Drug Facts
cc178825-1a4e-6bca-a00d-aa28d5abe589
HUMAN OTC DRUG LABEL
Sep 19, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Olopatadine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
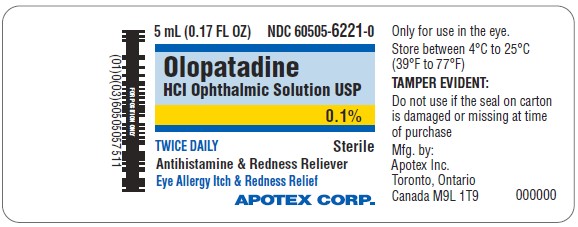
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
TWICE DAILY RELIEF
Olopatadine HCl Ophthalmic Solution, USP 0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
NDC 60505-6221-0

INDICATIONS & USAGE SECTION
Uses
Temporarily relieves itchy and red eyes due to pollen, ragweed, grass, animal hair and dander.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient** (in each tablet)**
Olopatadine (01.%) (equivalent to olopatadine hydrochloride 0.111%)
OTC - PURPOSE SECTION
Purpose
Antihistamine and redness reliever
WARNINGS SECTION
Warnings
For external use only
Do not use
- If solution changes color or becomes cloudy
- If you are sensitive to any ingredient in this product
- To treat contact lens related irritation
When using this product
- Do not touch tip of container to any surface to avoid contamination
- Remove contact lenses before use
- Wait at least 10 minutes before reinserting contact lenses after use
- Do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience**:**
- Eye pain
- Changes in vision
- Increased redness of the eye
- Itching worsens or lasts for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*adults and children 2 years and older: * put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day * if using other ophthalmic products while using this product, wait at least 5 minutes between each product * replace cap after each use *children under 2 years of age:
Consult a doctor
SPL UNCLASSIFIED SECTION
Other information
- Only for use in the eye
- Store between 4ºC to 25ºC (39ºF to 77ºF)
INACTIVE INGREDIENT SECTION
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate (anhydrous), hydrochloric acid and/or sodium hydroxide (to adjust pH), purified water and sodium chloride
OTC - QUESTIONS SECTION
Questions?
1-800-706-5575
