Metronidazole Topical Gel
METRONIDAZOLE TOPICAL GEL, 0.75%- metronidazole gelPrasco LaboratoriesFOR TOPICAL USE ONLY (NOT FOR OPHTHALMIC USE)
49b16ff1-299d-4dd0-82a7-fdb9c6e7c7a0
HUMAN PRESCRIPTION DRUG LABEL
Mar 1, 2024
Prasco Laboratories
DUNS: 065969375
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
metronidazole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
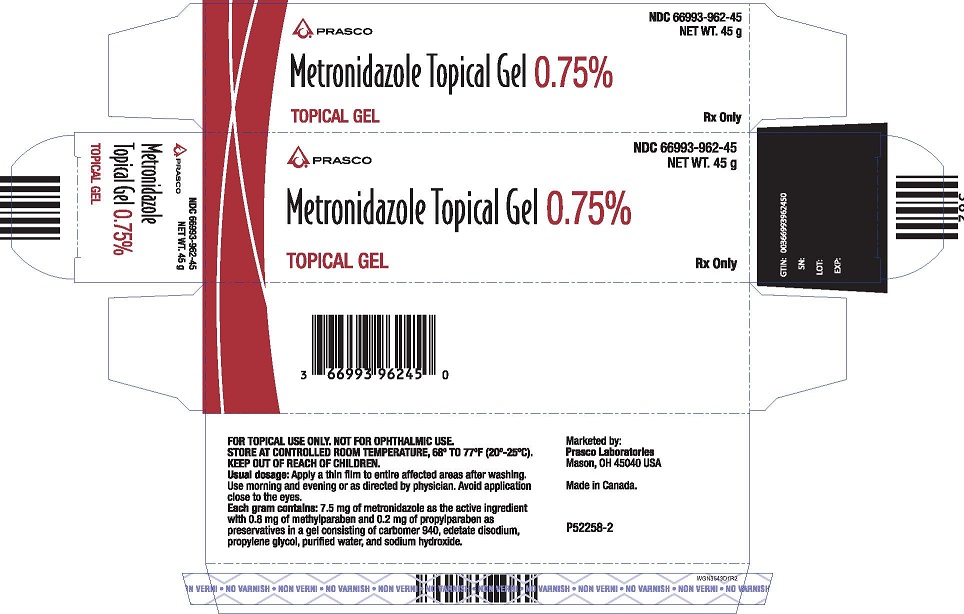
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - 45g Carton
Prasco
NDC 66993-962-45
NET WT. 45 g
Metronidazole Topical Gel 0.75%
TOPICAL GEL
Rx Only
FOR TOPICAL USE ONLY. NOT FOR OPTHALMIC USE.
STORE AT CONTROLLED ROOM TEMPERATURE, 68° to 77°F (20° - 25°C).
KEEP OUT OF REACH OF CHILDREN.
Ususal dosage: Apply a thin film to the entire affected areas after washing. Use morning and evening or as directed by physician. Avoid application close to the eyes.
Each gram contains: 7.5 mg of metronidazole as the active ingredient with 0.8 mg of methylparaben and 0.2 mg of propylparaben as preservatives in a gel consisting of carbomer 940, edetate disodium, propylene glycol, purified water, and sodium hydroxide.
Marketed By:
Prasco Laboratories
Mason, OH 45040 USA
Made in Canada.
P52258-2
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE:
Metronidazole Topical Gel is indicated for topical application in the treatment of inflammatory papules and pustules of rosacea.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS:
Metronidazole Topical Gel is contraindicated in individuals with a history of hypersensitivity to metronidazole, parabens, or other ingredients of the formulation.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS:
The following adverse experiences have been reported with the topical use of metronidazole: burning, skin irritation, dryness, transient redness, metallic taste, tingling or numbness of extremities and nausea.
DESCRIPTION SECTION
DESCRIPTION:
Metronidazole Topical Gel contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75%) in a gel consisting of carbomer 940, edetate disodium, methylparaben, propylene glycol, propylparaben, purified water, and sodium hydroxide. Metronidazole is classified therapeutically as an antiprotozoal and antibacterial agent. Chemically, metronidazole is named 2-methyl-5-nitro-1H-imidazole-1-ethanol and has the following structure:

CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY:
Bioavailability studies on the topical administration of 1 gram of Metronidazole Topical Gel (7.5 mg of metronidazole) to the face of 10 rosacea patients showed a maximum serum concentration of 66 nanograms per milliliter in one patient. This concentration is approximately 100 times less than concentrations afforded by a single 250 mg oral tablet. The serum metronidazole concentrations were below the detectable limits of the assay at the majority of time points in all patients. Three of the patients had no detectable serum concentrations of metronidazole at any time point. The mean dose of gel applied during clinical studies was 600 mg which represents 4.5 mg of metronidazole per application. Therefore, under normal usage levels, the formulation affords minimal serum concentrations of metronidazole. The mechanisms by which Metronidazole Topical Gel acts in the treatment of rosacea are unknown, but appear to include an anti-inflammatory effect.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION:
Apply and rub in a thin film of Metronidazole Topical Gel twice daily, morning and evening, to entire affected areas after washing.
Areas to be treated should be cleansed before application of Metronidazole Topical Gel. Patients may use cosmetics after application of Metronidazole Topical Gel.
PRECAUTIONS SECTION
PRECAUTIONS:
General:
Metronidazole Topical Gel has been reported to cause tearing of the eyes. Therefore, contact with the eyes should be avoided. If a reaction suggesting local irritation occurs, patients should be directed to use the medication less frequently or discontinue use. Metronidazole is a nitroimidazole and should be used with care in patients with evidence of, or history of blood dyscrasia.
Information for patients:
This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
Drug interactions:
Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin resulting in a prolongation of prothrombin time. The effect of topical metronidazole on prothrombin time is not known.
Carcinogenesis, mutagenesis, impairment of fertility:
Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic, oral administration in mice and rats but not in studies involving hamsters.
Metronidazole has shown evidence of mutagenic activity in several in vitro bacterial assay systems. In addition, a dose-response increase in the frequency of micronuclei was observed in mice after intraperitoneal injections and an increase in chromosome aberrations have been reported in patients with Crohn’s disease who were treated with 200-1200 mg/day of metronidazole for 1 to 24 months. However, no excess chromosomal aberrations in circulating human lymphocytes have been observed in patients treated for 8 months.
Pregnancy:
Teratogenic effects: Pregnancy category B: There has been no experience to date with the use of Metronidazole Topical Gel in pregnant patients. Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. No fetotoxicity was observed after oral metronidazole in rats or mice. However, because animal reproduction studies are not always predictive of human response and since oral metronidazole has been shown to be a carcinogen in some rodents, this drug should be used during pregnancy only if clearly needed.
Nursing mothers:
After oral administration, metronidazole is secreted in breast milk in concentrations similar to those found in the plasma. Even though Metronidazole Topical Gel blood levels are significantly lower than those achieved after oral metronidazole, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric use:
Safety and effectiveness in pediatric patients have not been established.
HOW SUPPLIED SECTION
HOW SUPPLIED:
Metronidazole Topical Gel is supplied in a 45 g aluminum tube –NDC 66993-962-45.
Storage conditions:
STORE AT CONTROLLED ROOM TEMPERATURE: 68° to 77°F (20° to 25°C).
Marketed by:
Prasco Laboratories
Mason, OH 45040 USA
P52259-1
Revised: September 2014
