NU-DERM system NORMAL-oily
OBAGI NU-DERM SYSTEM
48a6f977-d7b8-4da4-8bab-b3b8df5347c3
HUMAN PRESCRIPTION DRUG LABEL
Jan 26, 2023
OBAGI COSMECEUTICAL LLC
DUNS: 790553353
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Hydroquinone, Homosalate, Octisalate, and Zinc Oxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
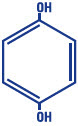
Hydroquinone, USP 4% is 1, 4-benzenediol. The drug is freely soluble in water and in alcohol. Chemically, hydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6H6O2; molecular weight is 110.11 g/mol. The chemical structure is in the diagram below.
C6H6O2
Each gram of Obagi Nu-Derm**®**** Clear contains:**
ACTIVE: Hydroquinone, USP 4% (40 mg/g)
INACTIVES: ascorbic acid, BHT, butylparaben, cetyl alcohol, disodium EDTA, glycerin, lactic acid, methylparaben, propylparaben, saponins, sodium lauryl sulfate, sodium metabisulfite, stearyl alcohol, tocopheryl acetate, water (aqua)
Each gram of Obagi Nu-Derm Blender**®**** contains:**
ACTIVE: Hydroquinone, USP 4% (40 mg/g)
INACTIVES: ascorbic acid, BHT, cetyl alcohol, disodium EDTA, glycerin, lactic acid, methylparaben, phenyl trimethicone, PPG-2 myristyl ether propionate, propylparaben, saponins, sodium lauryl sulfate, sodium metabisulfite, TEA-salicylate, tocopheryl acetate, water (aqua)
Each gram of Obagi Nu-Derm**®**** Sunfader****®**** contains:**
ACTIVES: Hydroquinone, USP 4% (40mg/g); Octinoxate, USP 7.5%; Oxybenzone, USP 5.5%
INACTIVES: ascorbic acid, BHT, butylparaben, cetyl alcohol, disodium EDTA, glycerin, methylparaben, propylparaben, saponins, sodium lauryl sulfate, sodium metabisulfite, stearyl alcohol, tocopheryl acetate, water (aqua)
HOW SUPPLIED SECTION
HOW SUPPLIED
Obagi Nu-Derm**®**** Clear** is available as follows:
Net wt. 2 oz. (57 g) bottle
NDC 62032-101-36
Obagi Nu-Derm Blender**®** is available as follows:
Net wt. 2 oz. (57 g) bottle
NDC 62032-100-36
Net wt. 1 oz. (28 g) bottle
NDC 62032-100-10
Obagi Nu-Derm Sunfader**®** is available as follows:
Net wt. 2 oz. (57 g) bottle
NDC 62032-116-36
Store at controlled room temperature: 15° to 25°C (59° to 77°F). Keep out of direct sunlight.
