JARDIANCE
These highlights do not include all the information needed to use JARDIANCE safely and effectively. See full prescribing information for JARDIANCE. JARDIANCE (empagliflozin tablets), for oral useInitial U.S. Approval: 2014
942a82a4-cbbd-4e44-9965-8d03f767912b
HUMAN PRESCRIPTION DRUG LABEL
Jul 24, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Empagliflozin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Ketoacidosis
Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization have been identified in clinical trials and postmarketing surveillance in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors, including JARDIANCE. Fatal cases of ketoacidosis have been reported in patients taking JARDIANCE. In placebo-controlled trials of patients with type 1 diabetes, the risk of ketoacidosis was increased in patients who received SGLT2 inhibitors compared to patients who received placebo. JARDIANCE is not indicated for the treatment of patients with type 1 diabetes mellitus [see Indications and Usage (1)].
Patients treated with JARDIANCE who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with JARDIANCE may be present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is suspected, JARDIANCE should be discontinued, patient should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, fluid and carbohydrate replacement.
In many of the postmarketing reports, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized and institution of treatment was delayed because presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. In some but not all cases, factors predisposing to ketoacidosis such as insulin dose reduction, acute febrile illness, reduced caloric intake, surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
Before initiating JARDIANCE, consider factors in the patient history that may predispose to ketoacidosis including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse.
For patients who undergo scheduled surgery, consider temporarily discontinuing JARDIANCE for at least 3 days prior to surgery [see Clinical Pharmacology (12.2, 12.3)].
Consider monitoring for ketoacidosis and temporarily discontinuing JARDIANCE in other clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or post-surgery). Ensure risk factors for ketoacidosis are resolved prior to restarting JARDIANCE.
Educate patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue JARDIANCE and seek medical attention immediately if signs and symptoms occur.
5.2 Volume Depletion
JARDIANCE can cause intravascular volume depletion which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)]. There have been post-marketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including JARDIANCE. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating JARDIANCE in patients with one or more of these characteristics, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating JARDIANCE. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
5.3 Urosepsis and Pyelonephritis
There have been reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving JARDIANCE. Treatment with JARDIANCE increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6)].
5.4 Hypoglycemia
Insulin and insulin secretagogues are known to cause hypoglycemia. In adult patients, the risk of hypoglycemia may be increased when JARDIANCE is used in combination with insulin secretagogues (e.g., sulfonylurea) or insulin. In pediatric patients aged 10 years and older, the risk of hypoglycemia was higher with JARDIANCE regardless of insulin use [see Adverse Reactions (6.1)].
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin. Inform patients using these concomitant medications and pediatric patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
5.5 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in patients with diabetes mellitus receiving SGLT2 inhibitors, including JARDIANCE. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with JARDIANCE presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue JARDIANCE, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.6 Genital Mycotic Infections
JARDIANCE increases the risk for genital mycotic infections [see Adverse Reactions (6.1)]. Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop genital mycotic infections. Monitor and treat as appropriate.
5.7 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions (e.g., angioedema) in patients treated with JARDIANCE. If a hypersensitivity reaction occurs, discontinue JARDIANCE; treat promptly per standard of care, and monitor until signs and symptoms resolve. JARDIANCE is contraindicated in patients with hypersensitivity to empagliflozin or any of the excipients in JARDIANCE [see Contraindications (4)].
- Ketoacidosis: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level. If suspected, discontinue JARDIANCE, evaluate and treat promptly. Before initiating JARDIANCE, consider risk factors for ketoacidosis. Patients on JARDIANCE may require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis. (5.1)
- Volume Depletion: Before initiating JARDIANCE, assess volume status and renal function in patients with impaired renal function, elderly patients, or patients on loop diuretics. Monitor for signs and symptoms during therapy. (5.2)
- Urosepsis and Pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated (5.3)
- Hypoglycemia: Adult patients taking an insulin secretagogue or insulin may have an increased risk of hypoglycemia. In pediatric patients 10 years of age and older, the risk of hypoglycemia was higher regardless of insulin use. Consider lowering the dosage of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating JARDIANCE. (5.4)
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (5.5)
- Genital Mycotic Infections: Monitor and treat as appropriate (5.6)
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., angioedema) have occurred with JARDIANCE. If hypersensitivity reactions occur, discontinue JARDIANCE, treat promptly, and monitor until signs and symptoms resolve. (5.7)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
See Table 4 for clinically relevant interactions with JARDIANCE.
Table 4 Clinically Relevant Interactions with JARDIANCE|
Diuretics | |
|---|---|
|
Clinical Impact |
Coadministration of empagliflozin with diuretics resulted in increased urine volume and frequency of voids, which might enhance the potential for volume depletion. |
|
Intervention |
Before initiating JARDIANCE, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating JARDIANCE. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy. |
|
Insulin or Insulin Secretagogues | |
|
Clinical Impact |
The risk of hypoglycemia is increased when JARDIANCE is used in combination with insulin secretagogues (e.g., sulfonylurea) or insulin. |
|
Intervention |
Coadministration of JARDIANCE with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower dosages of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. |
|
Lithium | |
|
Clinical Impact |
Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
|
Intervention |
Monitor serum lithium concentration more frequently during JARDIANCE initiation and dosage changes. |
|
Positive Urine Glucose Test | |
|
Clinical Impact |
SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. |
|
Intervention |
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
|
Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
|
Clinical Impact |
Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
|
Intervention |
Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control. |
See full prescribing information for information on drug interactions and interference of JARDIANCE with laboratory tests. (7)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Empagliflozin is an inhibitor of SGLT2, the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Empagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions such as lowering both pre- and afterload of the heart and downregulating sympathetic activity.
12.2 Pharmacodynamics
Urinary Glucose Excretion
In patients with type 2 diabetes mellitus, urinary glucose excretion increased immediately following a dose of empagliflozin and was maintained at the end of a 4-week treatment period averaging at approximately 64 grams per day with 10 mg empagliflozin and 78 grams per day with 25 mg empagliflozin once daily [see Clinical Studies (14)]. Data from single oral doses of empagliflozin in healthy subjects indicate that, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days for the 10 mg and 25 mg doses.
Urinary Volume
In a 5-day study, mean 24-hour urine volume increase from baseline was 341 mL on Day 1 and 135 mL on Day 5 of empagliflozin 25 mg once daily treatment.
Cardiac Electrophysiology
In a randomized, placebo-controlled, active-comparator, crossover study, 30 healthy subjects were administered a single oral dose of empagliflozin 25 mg, empagliflozin 200 mg (8 times the maximum dose), moxifloxacin, and placebo. No increase in QTc was observed with either 25 mg or 200 mg empagliflozin.
12.3 Pharmacokinetics
The pharmacokinetics of empagliflozin has been characterized in healthy volunteers and patients with type 2 diabetes mellitus and no clinically relevant differences were noted between the two populations. The steady-state mean plasma AUC and Cmax were 1,870 nmol∙h/L and 259 nmol/L, respectively, with 10 mg empagliflozin once daily treatment, and 4,740 nmol∙h/L and 687 nmol/L, respectively, with 25 mg empagliflozin once daily treatment. Systemic exposure of empagliflozin increased in a dose-proportional manner in the therapeutic dose range. Empagliflozin does not appear to have time-dependent pharmacokinetic characteristics. Following once-daily dosing, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state.
Absorption
After oral administration, peak plasma concentrations of empagliflozin were reached at 1.5 hours post-dose.
Administration of 25 mg empagliflozin after intake of a high-fat and high- calorie meal resulted in slightly lower exposure; AUC decreased by approximately 16% and Cmax decreased by approximately 37%, compared to fasted condition. The observed effect of food on empagliflozin pharmacokinetics was not considered clinically relevant and empagliflozin may be administered with or without food.
Distribution
The apparent steady-state volume of distribution was estimated to be 73.8 L based on a population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, the red blood cell partitioning was approximately 36.8% and plasma protein binding was 86.2%.
Elimination
The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 h and apparent oral clearance was 10.6 L/h based on the population pharmacokinetic analysis.
Metabolism
No major metabolites of empagliflozin were detected in human plasma and the most abundant metabolites were three glucuronide conjugates (2-O-, 3-O-, and 6-O-glucuronide). Systemic exposure of each metabolite was less than 10% of total drug-related material. In vitro studies suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9.
Excretion
Following administration of an oral [14C]-empagliflozin solution to healthy subjects, approximately 95.6% of the drug-related radioactivity was eliminated in feces (41.2%) or urine (54.4%). The majority of drug-related radioactivity recovered in feces was unchanged parent drug and approximately half of drug- related radioactivity excreted in urine was unchanged parent drug.
Specific Populations
Pediatric Patients
The pharmacokinetics and pharmacodynamics of empagliflozin were investigated in pediatric patients aged 10 to 17 years with type 2 diabetes mellitus. Oral administration of empagliflozin at 10 mg and 25 mg resulted in exposure within the range observed in adult patients.
Effects of Age, Body Mass Index, Gender, and Race
Age, body mass index (BMI), gender and race (Asians versus primarily Whites) do not have a clinically meaningful effect on pharmacokinetics of empagliflozin.
Patients with Hepatic Impairment
In adult patients with mild, moderate, and severe hepatic impairment according to the Child-Pugh classification, AUC of empagliflozin increased by approximately 23%, 47%, and 75%, and Cmax increased by approximately 4%, 23%, and 48%, respectively, compared to subjects with normal hepatic function.
Patients with Renal Impairment
In adult patients with type 2 diabetes mellitus with mild (eGFR: 60 to less than 90 mL/min/1.73 m2), moderate (eGFR: 30 to less than 60 mL/min/1.73 m2), and severe (eGFR: less than 30 mL/min/1.73 m2) renal impairment and patients on dialysis due to kidney failure, AUC of empagliflozin increased by approximately 18%, 20%, 66%, and 48%, respectively, compared to subjects with normal renal function. Peak plasma levels of empagliflozin were similar in patients with moderate renal impairment and patients on dialysis due to kidney failure compared to subjects with normal renal function. Peak plasma levels of empagliflozin were roughly 20% higher in patients with mild and severe renal impairment as compared to patients with normal renal function. Population pharmacokinetic analysis showed that the apparent oral clearance of empagliflozin decreased, with a decrease in eGFR leading to an increase in drug exposure. However, the fraction of empagliflozin that was excreted unchanged in urine, and urinary glucose excretion, declined with decrease in eGFR.
Drug Interaction Studies
In vitro Assessment of Drug Interactions
Empagliflozin does not inhibit, inactivate, or induce CYP450 isoforms. In vitro data suggest that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT1A3, UGT1A8, UGT1A9, and UGT2B7. Empagliflozin does not inhibit UGT1A1, UGT1A3, UGT1A8, UGT1A9, or UGT2B7. Therefore, no effect of empagliflozin is anticipated on concomitantly administered drugs that are substrates of the major CYP450 isoforms or UGT1A1, UGT1A3, UGT1A8, UGT1A9, or UGT2B7. The effect of UGT induction (e.g., induction by rifampicin or any other UGT enzyme inducer) on empagliflozin exposure has not been evaluated.
Empagliflozin is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), but it does not inhibit these efflux transporters at therapeutic doses. Based on in vitro studies, empagliflozin is considered unlikely to cause interactions with drugs that are P-gp substrates. Empagliflozin is a substrate of the human uptake transporters OAT3, OATP1B1, and OATP1B3, but not OAT1 and OCT2. Empagliflozin does not inhibit any of these human uptake transporters at clinically relevant plasma concentrations and, therefore, no effect of empagliflozin is anticipated on concomitantly administered drugs that are substrates of these uptake transporters.
In vivo Assessment of Drug Interactions
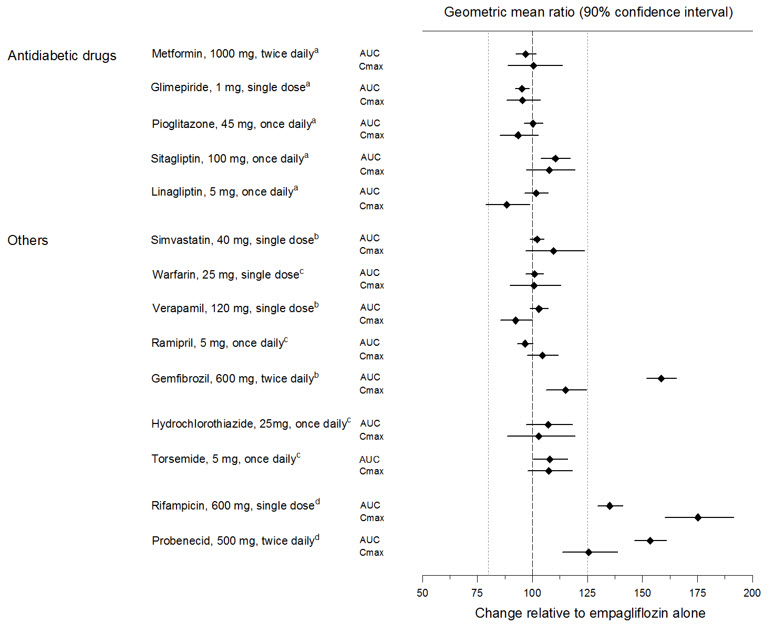
Empagliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, verapamil, ramipril, and simvastatin in healthy volunteers and with or without coadministration of hydrochlorothiazide and torsemide in patients with type 2 diabetes mellitus (see Figure 1). In subjects with normal renal function, coadministration of empagliflozin with probenecid resulted in a 30% decrease in the fraction of empagliflozin excreted in urine without any effect on 24-hour urinary glucose excretion. The relevance of this observation to patients with renal impairment is unknown.
Figure 1 Effect of Various Medications on the Pharmacokinetics of Empagliflozin as Displayed as 90% Confidence Interval of Geometric Mean AUC and Cmax Ratios [reference lines indicate 100% (80% - 125%)]|
aempagliflozin, 50 mg, once daily; bempagliflozin, 25 mg, single dose; cempagliflozin, 25 mg, once daily; dempagliflozin, 10 mg, single dose |
|
|
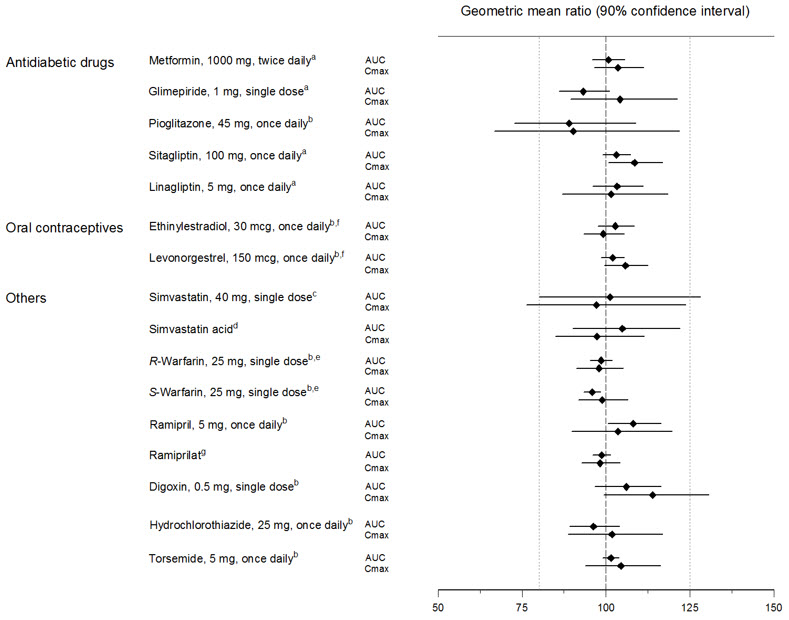
Empagliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, digoxin, ramipril, simvastatin, hydrochlorothiazide, torsemide, and oral contraceptives when coadministered in healthy volunteers (see Figure 2).
Figure 2 Effect of Empagliflozin on the Pharmacokinetics of Various Medications as Displayed as 90% Confidence Interval of Geometric Mean AUC and Cmax Ratios [reference lines indicate 100% (80% - 125%)]|
aempagliflozin, 50 mg, once daily; bempagliflozin, 25 mg, once daily; cempagliflozin, 25 mg, single dose; dadministered as simvastatin; eadministered as warfarin racemic mixture; fadministered as Microgynon®; gadministered as ramipril |
|
|
STORAGE AND HANDLING SECTION
Storage
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
JARDIANCE has been studied as monotherapy and in combination with metformin, sulfonylurea, pioglitazone, linagliptin, and insulin. JARDIANCE has also been studied in patients with type 2 diabetes mellitus with mild or moderate renal impairment.
In adult patients with type 2 diabetes mellitus, treatment with JARDIANCE reduced hemoglobin A1c (HbA1c), compared to placebo. The reduction in HbA1c for JARDIANCE compared with placebo was observed across subgroups including sex, race, geographic region, baseline BMI and duration of disease.
Monotherapy
A total of 986 patients with type 2 diabetes mellitus participated in a double-blind, placebo-controlled trial to evaluate the efficacy of JARDIANCE monotherapy.
Treatment-naïve patients with inadequately controlled type 2 diabetes mellitus entered an open-label placebo run-in for 2 weeks. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7% and 10% were randomized to placebo, JARDIANCE 10 mg, JARDIANCE 25 mg, or a reference comparator.
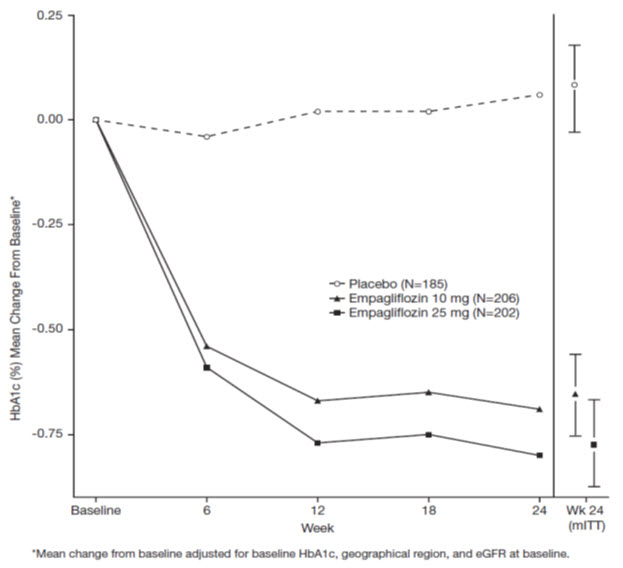
At Week 24, treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), fasting plasma glucose (FPG), and body weight compared with placebo (see Table 5 and Figure 3).
Table 5 Results at Week 24 From a Placebo-Controlled Monotherapy Trial of JARDIANCE|
JARDIANCE |
JARDIANCE |
Placebo | |
|---|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 24. At Week 24, 9.4%, 9.4%, and 30.7% was
imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and
placebo, respectively. | |||
|
**HbA1c (%)**a | |||
|
Baseline (mean) |
7.9 |
7.9 |
7.9 |
|
Change from baseline (adjusted mean) |
-0.7 |
-0.8 |
0.1 |
|
Difference from placebo (adjusted mean) (97.5% CI) |
-0.7b (-0.9, -0.6) |
-0.9b (-1.0, -0.7) |
-- |
|
Patients [n (%)] achieving HbA1c <7% |
72 (35%) |
88 (44%) |
25 (12%) |
|
**FPG (mg/dL)**c | |||
|
Baseline (mean) |
153 |
153 |
155 |
|
Change from baseline (adjusted mean) |
-19 |
-25 |
12 |
|
Difference from placebo (adjusted mean) (95% CI) |
-31 (-37, -26) |
-36 (-42, -31) |
-- |
|
Body Weight | |||
|
Baseline (mean) in kg |
78 |
78 |
78 |
|
% change from baseline (adjusted mean) |
-2.8 |
-3.2 |
-0.4 |
|
Difference from placebo (adjusted mean) (95% CI) |
-2.5b (-3.1, -1.9) |
-2.8b (-3.4, -2.2) |
-- |
|
|
At Week 24, the systolic blood pressure was statistically significantly reduced compared to placebo by -2.6 mmHg (placebo-adjusted, p-value=0.0231) in patients randomized to 10 mg of JARDIANCE and by -3.4 mmHg (placebo-corrected, p-value=0.0028) in patients randomized to 25 mg of JARDIANCE.
Add-On Combination Therapy with Metformin
A total of 637 patients with type 2 diabetes mellitus participated in a double-blind, placebo-controlled trial to evaluate the efficacy of JARDIANCE in combination with metformin.
Patients with type 2 diabetes mellitus inadequately controlled on at least 1,500 mg of metformin per day entered an open-label 2-week placebo run-in. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7% and 10% were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
At Week 24, treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 6).
Table 6 Results at Week 24 From a Placebo-Controlled Trial for JARDIANCE used in Combination with Metformin|
JARDIANCE |
JARDIANCE |
Placebo | |
|---|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 24. At Week 24, 9.7%, 14.1%, and 24.6% was
imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and
placebo, respectively. | |||
|
**HbA1c (%)**a | |||
|
Baseline (mean) |
7.9 |
7.9 |
7.9 |
|
Change from baseline (adjusted mean) |
-0.7 |
-0.8 |
-0.1 |
|
Difference from placebo + metformin (adjusted mean) (95% CI) |
-0.6b (-0.7, -0.4) |
-0.6b (-0.8, -0.5) |
-- |
|
Patients [n (%)] achieving HbA1c <7% |
75 (38%) |
74 (39%) |
23 (13%) |
|
**FPG (mg/dL)**c | |||
|
Baseline (mean) |
155 |
149 |
156 |
|
Change from baseline (adjusted mean) |
-20 |
-22 |
6 |
|
Difference from placebo + metformin (adjusted mean) |
-26 |
-29 |
-- |
|
Body Weight | |||
|
Baseline mean in kg |
82 |
82 |
80 |
|
% change from baseline (adjusted mean) |
-2.5 |
-2.9 |
-0.5 |
|
Difference from placebo (adjusted mean) (95% CI) |
-2.0b (-2.6, -1.4) |
-2.5b (-3.1, -1.9) |
-- |
At Week 24, the systolic blood pressure was statistically significantly reduced compared to placebo by -4.1 mmHg (placebo-corrected, p-value <0.0001) for JARDIANCE 10 mg and -4.8 mmHg (placebo-corrected, p-value <0.0001) for JARDIANCE 25 mg.
Initial Combination Therapy with Metformin
A total of 1,364 patients with type 2 diabetes mellitus participated in a double-blind, randomized, active-controlled trial to evaluate the efficacy of JARDIANCE in combination with metformin as initial therapy compared to the corresponding individual components.
Treatment-naïve patients with inadequately controlled type 2 diabetes mellitus entered an open-label placebo run-in for 2 weeks. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7% and 10.5% were randomized to one of 8 active-treatment arms: JARDIANCE 10 mg or 25 mg; metformin 1,000 mg, or 2,000 mg; JARDIANCE 10 mg in combination with 1,000 mg or 2,000 mg metformin; or JARDIANCE 25 mg in combination with 1,000 mg or 2,000 mg metformin.
At Week 24, initial therapy of JARDIANCE in combination with metformin provided statistically significant reductions in HbA1c (p-value <0.01) compared to the individual components (see Table 7).
Table 7 Glycemic Parameters at 24 Weeks in a Trial Comparing JARDIANCE and Metformin to the Individual Components as Initial Therapy|
JARDIANCE |
JARDIANCE |
JARDIANCE |
JARDIANCE |
JARDIANCE |
JARDIANCE |
Metformin |
Metformin | |
|---|---|---|---|---|---|---|---|---|
|
aMetformin total daily dose, administered in two equally divided doses per
day. | ||||||||
|
HbA1c (%) | ||||||||
|
Baseline (mean) |
8.7 |
8.7 |
8.8 |
8.7 |
8.6 |
8.9 |
8.7 |
8.6 |
|
Change from baseline (adjusted mean) |
-2.0 |
-2.1 |
-1.9 |
-2.1 |
-1.4 |
-1.4 |
-1.2 |
-1.8 |
|
Comparison vs JARDIANCE (adjusted mean) (95% CI) |
-0.6b |
-0.7b |
-0.6c |
-0.7c |
-- |
-- |
-- |
-- |
|
Comparison vs metformin (adjusted mean) (95% CI) |
-0.8b |
-0.3b |
-0.8c |
-0.3c |
-- |
-- |
-- |
-- |
Add-On Combination Therapy with Metformin and Sulfonylurea
A total of 666 patients with type 2 diabetes mellitus participated in a double-blind, placebo-controlled trial to evaluate the efficacy of JARDIANCE in combination with metformin plus a sulfonylurea.
Patients with inadequately controlled type 2 diabetes mellitus on at least 1,500 mg per day of metformin and on a sulfonylurea, entered a 2-week open- label placebo run-in. At the end of the run-in, patients who remained inadequately controlled and had an HbA1c between 7% and 10% were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
Treatment with JARDIANCE 10 mg or 25 mg daily provided statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 8).
Table 8 Results at Week 24 from a Placebo-Controlled Trial for JARDIANCE in Combination with Metformin and Sulfonylurea|
JARDIANCE |
JARDIANCE |
Placebo | |
|---|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 24. At Week 24, 17.8%, 16.7%, and 25.3%
was imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and
placebo, respectively. | |||
|
**HbA1c (%)**a | |||
|
Baseline (mean) |
8.1 |
8.1 |
8.2 |
|
Change from baseline (adjusted mean) |
-0.8 |
-0.8 |
-0.2 |
|
Difference from placebo (adjusted mean) (95% CI) |
-0.6b (-0.8, -0.5) |
-0.6b (-0.7, -0.4) |
-- |
|
Patients [n (%)] achieving HbA1c <7% |
55 (26%) |
65 (32%) |
20 (9%) |
|
**FPG (mg/dL)**c | |||
|
Baseline (mean) |
151 |
156 |
152 |
|
Change from baseline (adjusted mean) |
-23 |
-23 |
6 |
|
Difference from placebo (adjusted mean) |
-29 |
-29 |
-- |
|
Body Weight | |||
|
Baseline mean in kg |
77 |
78 |
76 |
|
% change from baseline (adjusted mean) |
-2.9 |
-3.2 |
-0.5 |
|
Difference from placebo (adjusted mean) (95% CI) |
-2.4b (-3.0, -1.8) |
-2.7b (-3.3, -2.1) |
-- |
In Combination with Linagliptin as Add-On to Metformin Therapy
A total of 686 patients with type 2 diabetes mellitus participated in a double-blind, active-controlled trial to evaluate the efficacy of JARDIANCE 10 mg or 25 mg in combination with linagliptin 5 mg compared to the individual components.
Patients with type 2 diabetes mellitus inadequately controlled on at least 1,500 mg of metformin per day entered a single-blind placebo run-in period for 2 weeks. At the end of the run-in period, patients who remained inadequately controlled and had an HbA1c between 7% and 10.5% were randomized 1:1:1:1:1 to one of 5 active-treatment arms of JARDIANCE 10 mg or 25 mg, linagliptin 5 mg, or linagliptin 5 mg in combination with 10 mg or 25 mg JARDIANCE as a fixed- dose combination tablet.
At Week 24, JARDIANCE 10 mg or 25 mg used in combination with linagliptin 5 mg provided statistically significant improvement in HbA1c (p-value <0.0001) and FPG (p-value <0.001) compared to the individual components in patients who had been inadequately controlled on metformin. Treatment with JARDIANCE/linagliptin 25 mg/5 mg or JARDIANCE/linagliptin 10 mg/5 mg daily also resulted in a statistically significant reduction in body weight compared to linagliptin 5 mg (p-value <0.0001). There was no statistically significant difference in body weight compared to JARDIANCE alone.
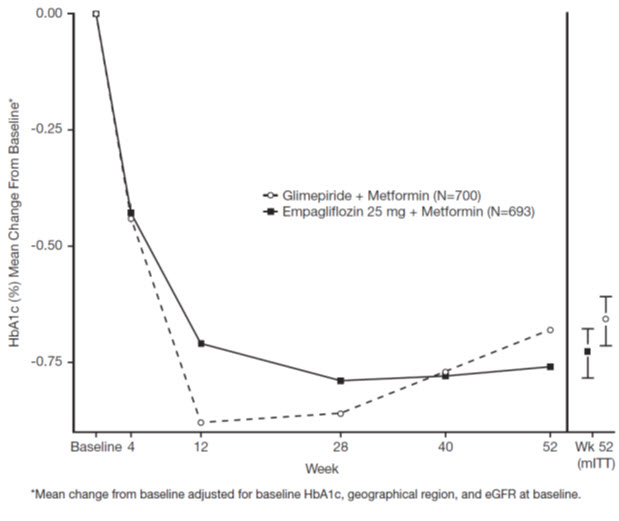
Active-Controlled Trial versus Glimepiride in Combination with Metformin
The efficacy of JARDIANCE was evaluated in a double-blind, glimepiride- controlled, trial in 1,545 patients with type 2 diabetes mellitus with insufficient glycemic control despite metformin therapy.
Patients with inadequate glycemic control and an HbA1c between 7% and 10% after a 2-week run-in period were randomized to glimepiride or JARDIANCE 25 mg.
At Week 52, JARDIANCE 25 mg and glimepiride lowered HbA1c and FPG (see Table 9, Figure 4). The difference in observed effect size between JARDIANCE 25 mg and glimepiride excluded the pre-specified non-inferiority margin of 0.3%. The mean daily dosage of glimepiride was 2.7 mg and the maximal approved dosage in the United States is 8 mg per day.
Table 9 Results at Week 52 from an Active-Controlled Trial Comparing JARDIANCE to Glimepiride as Add-On Therapy in Patients Inadequately Controlled on Metformin|
JARDIANCE 25 mg |
Glimepiride | |
|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute data missing at Week 52. At Week 52, data was imputed for 15.3%
and 21.9% of patients randomized to JARDIANCE 25 mg and glimepiride,
respectively. | ||
|
**HbA1c (%)**a | ||
|
Baseline (mean) |
7.9 |
7.9 |
|
Change from baseline (adjusted mean) |
-0.7 |
-0.7 |
|
Difference from glimepiride (adjusted mean) (97.5% CI) |
-0.07b (-0.15, 0.01) |
-- |
|
**FPG (mg/dL)**d | ||
|
Baseline (mean) |
150 |
150 |
|
Change from baseline (adjusted mean) |
-19 |
-9 |
|
Difference from glimepiride (adjusted mean) |
-11 |
-- |
|
Body Weight | ||
|
Baseline mean in kg |
82.5 |
83 |
|
% change from baseline (adjusted mean) |
-3.9 |
2.0 |
|
Difference from glimepiride (adjusted mean) (95% CI) |
-5.9c (-6.3, -5.5) |
-- |
|
|
At Week 52, the adjusted mean change from baseline in systolic blood pressure was -3.6 mmHg, compared to 2.2 mmHg for glimepiride. The differences between treatment groups for systolic blood pressure was statistically significant (p-value <0.0001).
At Week 104, the adjusted mean change from baseline in HbA1c was -0.75% for JARDIANCE 25 mg and -0.66% for glimepiride. The adjusted mean treatment difference was -0.09% with a 97.5% confidence interval of (-0.32%, 0.15%), excluding the pre-specified non-inferiority margin of 0.3%. The mean daily dosage of glimepiride was 2.7 mg and the maximal approved dosage in the United States is 8 mg per day. The Week 104 analysis included data with and without concomitant glycemic rescue medication, as well as off-treatment data. Missing data for patients not providing any information at the visit were imputed based on the observed off-treatment data. In this multiple imputation analysis, 13.9% of the data were imputed for JARDIANCE 25 mg and 12.9% for glimepiride.
At Week 104, JARDIANCE 25 mg daily resulted in a statistically significant difference in change from baseline for body weight compared to glimepiride (-3.1 kg for JARDIANCE 25 mg vs. +1.3 kg for glimepiride; ANCOVA-LOCF, p-value <0.0001).
Add-On Combination Therapy with Pioglitazone with or without Metformin
A total of 498 patients with type 2 diabetes mellitus participated in a double-blind, placebo-controlled trial to evaluate the efficacy of JARDIANCE in combination with pioglitazone, with or without metformin.
Patients with inadequately controlled type 2 diabetes mellitus on metformin at a dose of at least 1,500 mg per day and pioglitazone at a dose of at least 30 mg per day were placed into an open-label placebo run-in for 2 weeks. Patients with inadequate glycemic control and an HbA1c between 7% and 10% after the run-in period were randomized to placebo, JARDIANCE 10 mg, or JARDIANCE 25 mg.
Treatment with JARDIANCE 10 mg or 25 mg daily resulted in statistically significant reductions in HbA1c (p-value <0.0001), FPG, and body weight compared with placebo (see Table 10).
Table 10 Results of Placebo-Controlled Trial for JARDIANCE in Combination Therapy with Pioglitazone|
JARDIANCE |
JARDIANCE |
Placebo | |
|---|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 24. At Week 24, 10.9%, 8.3%, and 20.6% was
imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and
placebo, respectively. | |||
|
**HbA1c (%)**a | |||
|
Baseline (mean) |
8.1 |
8.1 |
8.2 |
|
Change from baseline (adjusted mean) |
-0.6 |
-0.7 |
-0.1 |
|
Difference from placebo + pioglitazone (adjusted mean) (95% CI) |
-0.5b (-0.7, -0.3) |
-0.6b (-0.8, -0.4) |
-- |
|
Patients [n (%)] achieving HbA1c <7% |
36 (24%) |
48 (30%) |
12 (8%) |
|
**FPG (mg/dL)**c | |||
|
Baseline (mean) |
152 |
152 |
152 |
|
Change from baseline (adjusted mean) |
-17 |
-22 |
7 |
|
Difference from placebo + pioglitazone (adjusted mean) (97.5% CI) |
-23b (-31.8, -15.2) |
-28b (-36.7, -20.2) |
-- |
|
Body Weight | |||
|
Baseline mean in kg |
78 |
79 |
78 |
|
% change from baseline (adjusted mean) |
-2.0 |
-1.8 |
0.6 |
|
Difference from placebo (adjusted mean) (95% CI) |
-2.6b (-3.4, -1.8) |
-2.4b (-3.2, -1.6) |
-- |
Add-On Combination with Insulin with or without Metformin and/or Sulfonylureas
A total of 494 patients with type 2 diabetes mellitus inadequately controlled on insulin, or insulin in combination with oral drugs participated in a double-blind, placebo-controlled trial to evaluate the efficacy of JARDIANCE as add-on therapy to insulin over 78 weeks.
Patients entered a 2-week placebo run-in period on basal insulin (e.g., insulin glargine, insulin detemir, or NPH insulin) with or without metformin and/or sulfonylurea background therapy. Following the run-in period, patients with inadequate glycemic control were randomized to the addition of JARDIANCE 10 mg, JARDIANCE 25 mg, or placebo. Patients were maintained on a stable dose of insulin prior to enrollment, during the run-in period, and during the first 18 weeks of treatment. For the remaining 60 weeks, insulin could be adjusted. The mean total daily insulin dose at baseline for JARDIANCE 10 mg, 25 mg, and placebo was 45 IU, 48 IU, and 48 IU, respectively.
JARDIANCE used in combination with insulin (with or without metformin and/or sulfonylurea) provided statistically significant reductions in HbA1c and FPG compared to placebo after both 18 and 78 weeks of treatment (see Table 11). JARDIANCE 10 mg or 25 mg daily also resulted in statistically significantly greater percent body weight reduction compared to placebo.
Table 11 Results at Week 18 and 78 for a Placebo-Controlled Trial for JARDIANCE in Combination with Insulin|
18 weeks |
78 weeks | |||||
|---|---|---|---|---|---|---|
|
JARDIANCE |
JARDIANCE |
Placebo |
JARDIANCE |
JARDIANCE |
Placebo | |
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 18 and 78. At Week 18, 21.3%, 30.3%, and
21.8% was imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg,
and placebo, respectively. At Week 78, 32.5%, 38.1% and 42.4% was imputed for
patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and placebo,
respectively. | ||||||
|
**HbA1c (%)**a | ||||||
|
Baseline (mean) |
8.3 |
8.3 |
8.2 |
8.3 |
8.3 |
8.2 |
|
Change from baseline (adjusted mean) |
-0.6 |
-0.7 |
0 |
-0.4 |
-0.6 |
0.1 |
|
Difference from placebo (adjusted mean) (97.5% CI) |
-0.6b |
-0.7b |
-- |
-0.5b |
-0.7b |
-- |
|
Patients (%) achieving HbA1c <7% |
18.0 |
19.5 |
5.5 |
12.0 |
17.5 |
6.7 |
|
FPG (mg/dL) | ||||||
|
Baseline (mean) |
138 |
146 |
142 |
138 |
146 |
142 |
|
Change from baseline (adjusted mean, SE) |
-17.9 (3.2) |
-19.1 (3.3) |
10.4 (3.1) |
-10.1 (3.2) |
-15.2 (3.4) |
2.8 (3.2) |
|
Difference from placebo (adjusted mean) (95% CI) |
-28.2b |
-29.5b |
-- |
-12.9c |
-17.9b |
-- |
|
Body Weight | ||||||
|
Baseline mean in kg |
92 |
95 |
90 |
92 |
95 |
90 |
|
% change from baseline (adjusted mean) |
-1.8 |
-1.4 |
-0.1 |
-2.4 |
-2.4 |
0.7 |
|
Difference from placebo (adjusted mean) (95% CI) |
-1.7d |
-1.3e |
-- |
-3.0b |
-3.0b |
-- |
Add-on Combination with MDI Insulin with or without Metformin
A total of 563 patients with type 2 diabetes mellitus inadequately controlled on multiple daily injections (MDI) of insulin (total daily dose >60 IU), alone or in combination with metformin, participated in a double-blind, placebo- controlled trial to evaluate the efficacy of JARDIANCE as add-on therapy to MDI insulin over 18 weeks.
Patients entered a 2-week placebo run-in period on MDI insulin with or without metformin background therapy. Following the run-in period, patients with inadequate glycemic control were randomized to the addition of JARDIANCE 10 mg, JARDIANCE 25 mg, or placebo. Patients were maintained on a stable dose of insulin prior to enrollment, during the run-in period, and during the first 18 weeks of treatment. The mean total daily insulin dose at baseline for JARDIANCE 10 mg, JARDIANCE 25 mg, and placebo was 88.6 IU, 90.4 IU, and 89.9 IU, respectively.
JARDIANCE 10 mg or 25 mg daily used in combination with MDI insulin (with or without metformin) provided statistically significant reductions in HbA1c compared to placebo after 18 weeks of treatment (see Table 12).
Table 12 Results at Week 18 for a Placebo-Controlled Trial for JARDIANCE in Combination with Insulin and with or without Metformin|
JARDIANCE 10 mg |
JARDIANCE 25 mg |
Placebo | |
|---|---|---|---|
|
aModified intent-to-treat population. Last observation on trial (LOCF) was
used to impute missing data at Week 18. At Week 18, 23.7%, 22.8% and 23.4% was
imputed for patients randomized to JARDIANCE 10 mg, JARDIANCE 25 mg, and
placebo, respectively. | |||
|
**HbA1c (%)**a | |||
|
Baseline (mean) |
8.4 |
8.3 |
8.3 |
|
Change from baseline (adjusted mean) |
-0.9 |
-1.0 |
-0.5 |
|
Difference from placebo (adjusted mean) (95% CI) |
-0.4b (-0.6, -0.3) |
-0.5b (-0.7, -0.4) |
-- |
During an extension period with treatment for up to 52 weeks, insulin could be adjusted to achieve defined glucose target levels. The change from baseline in HbA1c was maintained from 18 to 52 weeks with both JARDIANCE 10 mg and 25 mg. After 52 weeks, JARDIANCE 10 mg or 25 mg daily resulted in statistically greater percent body weight reduction compared to placebo (p-value <0.0001). The mean change in body weight from baseline was -1.95 kg for JARDIANCE 10 mg, and -2.04 kg for JARDIANCE 25 mg.
Renal Impairment
A total of 738 patients with type 2 diabetes mellitus and a baseline eGFR less than 90 mL/min/1.73 m2 participated in a randomized, double-blind, placebo- controlled, parallel-group trial to evaluate the efficacy of JARDIANCE in patients with type 2 diabetes mellitus and renal impairment. The trial population comprised of 290 patients with mild renal impairment (eGFR 60 to less than 90 mL/min/1.73 m2), 374 patients with moderate renal impairment (eGFR 30 to less than 60 mL/min/1.73 m2), and 74 with severe renal impairment (eGFR less than 30 mL/min/1.73 m2). A total of 194 patients with moderate renal impairment had a baseline eGFR of 30 to less than 45 mL/min/1.73 m2 and 180 patients had a baseline eGFR of 45 to less than 60 mL/min/1.73 m2.
At Week 24, JARDIANCE 25 mg provided statistically significant reduction in HbA1c relative to placebo in patients with mild to moderate renal impairment (see Table 13). A statistically significant reduction relative to placebo was also observed with JARDIANCE 25 mg in patients with either mild [-0.7 (95% CI: -0.9, -0.5)] or moderate [-0.4 (95% CI: -0.6, -0.3)] renal impairment and with JARDIANCE 10 mg in patients with mild [-0.5 (95% CI: -0.7, -0.3)] renal impairment.
The glucose lowering efficacy of JARDIANCE 25 mg decreased with decreasing level of renal function in the mild to moderate range. Least square mean HbA1c changes at 24 weeks were -0.6%, -0.5%, and -0.2% for those with a baseline eGFR of 60 to less than 90 mL/min/1.73 m2, 45 to less than 60 mL/min/1.73 m2, and 30 to less than 45 mL/min/1.73 m2, respectively [see Dosage and Administration (2) and Use in Specific Populations (8.6)]. For placebo, least square mean HbA1c changes at 24 weeks were 0.1%, -0.1%, and 0.2% for patients with a baseline eGFR of 60 to less than 90 mL/min/1.73 m2, 45 to less than 60 mL/min/1.73 m2, and 30 to less than 45 mL/min/1.73 m2, respectively.
Table 13 Results at Week 24 (LOCF) of Placebo-Controlled Trial for JARDIANCE in Adults with Type 2 Diabetes Mellitus and Renal Impairment|
Mild and Moderate Impairmentb | |
|---|---|
|
JARDIANCE 25 mg | |
|
ap-value <0.0001 (HbA1c: ANCOVA model includes baseline HbA1c, treatment,
renal function, and background medication) | |
|
HbA1c | |
|
Number of patients |
n=284 |
|
Comparison vs placebo (adjusted mean) (95% CI) |
-0.5a (-0.6, -0.4) |
For patients with severe renal impairment, the analyses of changes in HbA1c and FPG showed no discernible treatment effect of JARDIANCE 25 mg compared to placebo [see Indications and Usage (1), Dosage and Administration (2.1, 2.2) and Use in Specific Populations (8.6)].
14.2 Glycemic Control Trial in Pediatric Patients Aged 10 to 17 Years with
Type 2 Diabetes Mellitus
DINAMO (NCT03429543) was a 26-week, double-blind, randomized, placebo- controlled, parallel group trial, with a double-blind active treatment safety extension period up to 52 weeks to assess the efficacy of JARDIANCE. The trial enrolled pediatric patients aged 10 to 17 years with inadequately controlled type 2 diabetes mellitus (HbA1c 6.5 to 10.5%). Patients treated with metformin (at least 1,000 mg daily or maximally tolerated dose), with or without insulin therapy, and those with a history of intolerance to metformin therapy were enrolled. Patients were randomized to 3 treatment arms (JARDIANCE 10 mg, a dipeptidyl peptidase-4 (DPP-4) inhibitor or placebo), over 26 weeks. Patients in the JARDIANCE 10 mg group who failed to achieve HbA1c <7.0% at Week 12 underwent a second randomization at Week 14 to remain on the 10 mg dose or increase to 25 mg. Patients on placebo were re-randomized at Week 26 to one of the JARDIANCE doses (10 mg or 25 mg) or a DPP-4 inhibitor.
A total of 157 patients were treated with either JARDIANCE (10 mg or 25 mg; N=52), a DPP-4 inhibitor (N=52), or placebo (N=53). Background therapies as adjunct to diet and exercise included metformin (51%), a combination of metformin and insulin (40.1%), insulin (3.2%), or none (5.7%). The mean HbA1c at baseline was 8.0% and the mean duration of type 2 diabetes mellitus was 2.1 years. The mean age was 14.5 years (range: 10-17 years) and 51.6% were aged 15 years and older. Approximately, 50% were White, 6% were Asian, 31% were Black or African American, and 38% were of Hispanic or Latino ethnicity. The mean BMI was 36.0 kg/m2 and mean BMI Z-score was 3.0. Patients with an eGFR less than 60 mL/min/1.73 m2 were not enrolled in the trial. Approximately 25% of the study population had microalbuminuria or macroalbuminuria.
At Week 26, treatment with JARDIANCE was superior in reducing HbA1c from baseline versus placebo (see Table 14).
Table 14 Results at Week 26 for a Placebo-Controlled Trial for JARDIANCE in Combination with Metformin and/or Insulin or as Monotherapy in Pediatric Patients Aged 10 to 17 years with Type 2 Diabetes Mellitusa|
JARDIANCE |
Placebo | |
|---|---|---|
|
aModified intent-to-treat set (All randomized and treated patients with
baseline measurement). | ||
|
**HbA1c (%)**b | ||
|
Number of patients |
n=52 |
n=53 |
|
Baseline (mean) |
8.0 |
8.1 |
|
Change from baselinec |
-0.2 |
0.7 |
|
Difference from placeboc (95% CI) |
-0.8e (-1.5, -0.2) |
-- |
|
**FPG (mg/dL)**b,d | ||
|
Number of patients |
n=48 |
n=52 |
|
Baseline (mean) |
154 |
159 |
|
Change from baselinec |
-19 |
17 |
|
Difference from placeboc (95% CI) |
-36 (-60.7, -10.7) |
-- |
14.3 Cardiovascular Outcomes in Adults with Type 2 Diabetes Mellitus and
Atherosclerotic Cardiovascular Disease
The effect of JARDIANCE on cardiovascular (CV) risk in adult patients with type 2 diabetes mellitus and established, stable, atherosclerotic CV disease was evaluated in the EMPA-REG OUTCOME trial, a multicenter, multinational, randomized, double-blind parallel group trial. The trial compared the risk of experiencing a major adverse cardiovascular event (MACE) between JARDIANCE and placebo when these were added to and used concomitantly with standard of care treatments for diabetes mellitus and atherosclerotic CV disease. Concomitant antidiabetic medications were to be kept stable for the first 12 weeks of the trial. Thereafter, antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
A total of 7,020 patients were treated (JARDIANCE 10 mg = 2,345; JARDIANCE 25 mg = 2,342; placebo = 2,333) and followed for a median of 3.1 years. Approximately 72% of the trial population was White, 22% was Asian, and 5% was Black. The mean age was 63 years and approximately 72% were male.
All patients in the trial had inadequately controlled type 2 diabetes mellitus at baseline (HbA1c greater than or equal to 7%). The mean HbA1c at baseline was 8.1% and 57% of participants had diabetes mellitus for more than 10 years. Approximately 31%, 22% and 20% reported a past history of neuropathy, retinopathy and nephropathy to investigators, respectively and the mean eGFR was 74 mL/min/1.73 m2. At baseline, patients were treated with one (~30%) or more (~70%) antidiabetic medications including metformin (74%), insulin (48%), and sulfonylurea (43%).
All patients had established atherosclerotic CV disease at baseline including one (82%) or more (18%) of the following: a documented history of coronary artery disease (76%), stroke (23%) or peripheral artery disease (21%). At baseline, the mean systolic blood pressure was 136 mmHg, the mean diastolic blood pressure was 76 mmHg, the mean LDL was 86 mg/dL, the mean HDL was 44 mg/dL, and the mean urinary albumin to creatinine ratio (UACR) was 175 mg/g. At baseline, approximately 81% of patients were treated with renin angiotensin system inhibitors, 65% with beta-blockers, 43% with diuretics, 77% with statins, and 86% with antiplatelet agents (mostly aspirin).
The primary endpoint in EMPA-REG OUTCOME was the time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event was defined as occurrence of either a CV death or a non-fatal myocardial infarction (MI) or a non-fatal stroke. The statistical analysis plan had pre-specified that the 10 and 25 mg doses would be combined. A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE and superiority on MACE if non-inferiority was demonstrated. Type-1 error was controlled across multiples tests using a hierarchical testing strategy.
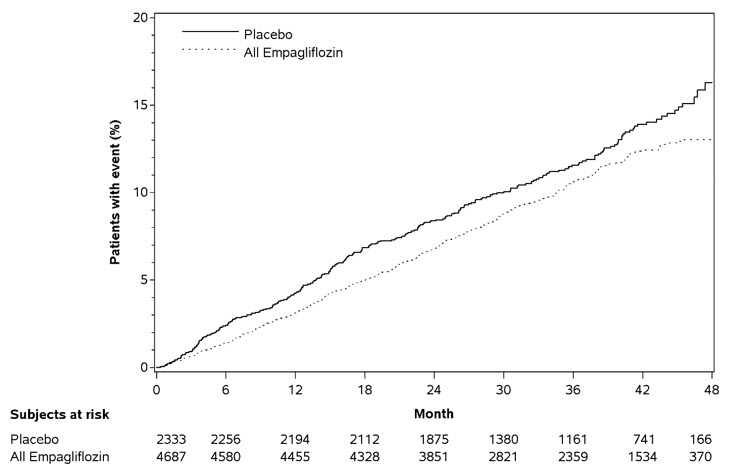
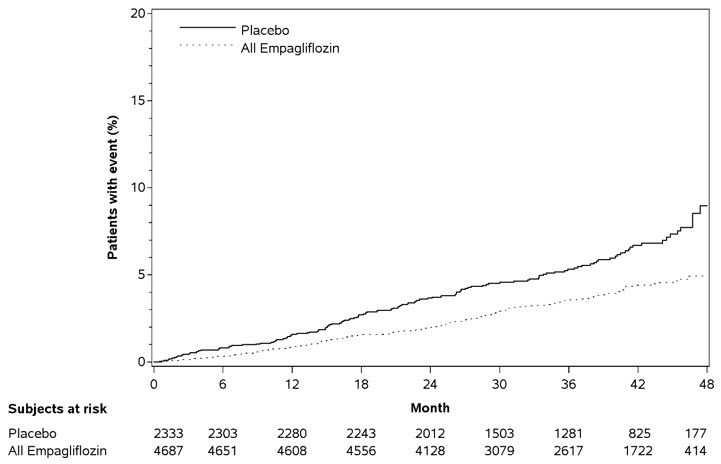
JARDIANCE significantly reduced the risk of first occurrence of primary composite endpoint of CV death, non-fatal myocardial infarction, or non-fatal stroke (HR: 0.86; 95% CI: 0.74, 0.99). The treatment effect was due to a significant reduction in the risk of CV death in subjects randomized to empagliflozin (HR: 0.62; 95% CI: 0.49, 0.77), with no change in the risk of non-fatal myocardial infarction or non-fatal stroke (see Table 15 and Figures 5 and 6). Results for the 10 mg and 25 mg empagliflozin dosages were consistent with results for the combined dosage groups.
Table 15 Treatment Effect for the Primary Composite Endpoint and its Componentsa|
Placebo |
JARDIANCE |
Hazard ratio vs placebo | |
|---|---|---|---|
|
aTreated set (patients who had received at least one dose of trial drug) | |||
|
Composite of CV death, non-fatal myocardial infarction, non-fatal stroke (time to first occurrence)b |
282 (12.1%) |
490 (10.5%) |
0.86 (0.74, 0.99) |
|
Non-fatal myocardial infarctionc |
121 (5.2%) |
213 (4.5%) |
0.87 (0.70, 1.09) |
|
Non-fatal strokec |
60 (2.6%) |
150 (3.2%) |
1.24 (0.92, 1.67) |
|
CV deathc |
137 (5.9%) |
172 (3.7%) |
0.62 (0.49, 0.77) |
|
|
|
|
The efficacy of JARDIANCE on CV death was generally consistent across major demographic and disease subgroups.
Vital status was obtained for 99.2% of subjects in the trial. A total of 463 deaths were recorded during the EMPA-REG OUTCOME trial. Most of these deaths were categorized as CV deaths. The non-CV deaths were only a small proportion of deaths and were balanced between the treatment groups (2.1% in patients treated with JARDIANCE, and 2.4% of patients treated with placebo).
14.4 Heart Failure Trials in Adults
EMPEROR-Reduced (NCT03057977) was a double-blind trial conducted in adults with chronic heart failure (New York Heart Association [NYHA] functional class II-IV) with left ventricular ejection fraction (LVEF) ≤40% to evaluate the efficacy of JARDIANCE as adjunct to standard of care heart failure therapy.
Of 3,730 patients, 1,863 were randomized to JARDIANCE 10 mg and 1,867 to placebo and were followed for a median of 16 months. The mean age of the trial population was 67 years (range: 25 to 94 years) and 76% were men, 24% were women, and 27% were 75 years of age or older. Approximately 71% of the trial population were White, 18% Asian and 7% Black or African American. At baseline, 50% of the patients had type 2 diabetes mellitus.
At randomization, 75% of patients were NYHA class II, 24% were class III and 0.5% were class IV. The mean LVEF was 28%. At baseline, the mean eGFR was 62 mL/min/1.73 m2 and the median urinary albumin to creatinine ratio (UACR) was 22 mg/g. Approximately half of the patients (52%) had eGFR equal to or above 60 mL/min/1.73 m2, 24% had eGFR 45 to less than 60 mL/min/1.73 m2, 19% had eGFR 30 to less than 45 mL/min/1.73 m2 and 5% had eGFR 20 to less than 30 mL/min/1.73 m2.
At baseline, 88% of patients were treated with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), or angiotensin receptor-neprilysin inhibitors (ARNI), 95% with beta-blockers, 71% with mineralocorticoid receptor antagonists (MRA), and 95% with diuretics.
The primary endpoint was the time to first event of either cardiovascular (CV) death or hospitalization for heart failure (HHF). First and recurrent HHF was assessed as a key secondary endpoint.
JARDIANCE was superior in reducing the risk of the primary composite endpoint of cardiovascular death or hospitalization for heart failure compared with placebo, mostly through a reduction in hospitalization for heart failure. JARDIANCE reduced the risk of first and recurrent HHF (see Table 16 and Figures 7 and 8).
Table 16 Treatment Effect for the Primary Composite Endpoint, its Components, and Key Secondary Endpoints|
Placebo |
JARDIANCE 10 mg |
Hazard ratio vs placebo |
p-value | |
|---|---|---|---|---|
|
Number of Patients (%) | ||||
|
aTime to first event | ||||
|
CV death or HHFa |
462 (24.7%) |
361 (19.4%) |
0.75 (0.65, 0.86) |
<0.0001 |
|
CV deatha,b |
202 (10.8%) |
187 (10.0%) |
0.92 (0.75, 1.12) | |
|
HHFa |
342 (18.3%) |
246 (13.2%) |
0.69 (0.59, 0.81) | |
|
Number of Events | ||||
|
First and recurrent HHFc |
553 |
388 |
0.70 (0.58, 0.85) |
0.0003 |
Figure 7 Time to First Occurrence of the Primary Composite Endpoint of CV Death or Hospitalization for Heart Failure
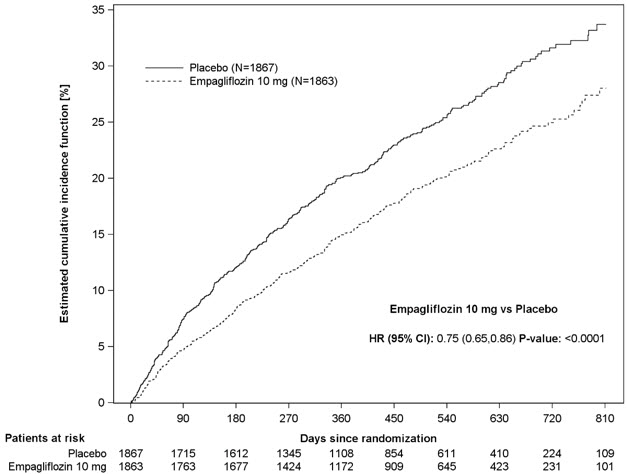
Figure 8 Time to Event of Hospitalization for Heart Failure (First and Recurrent)
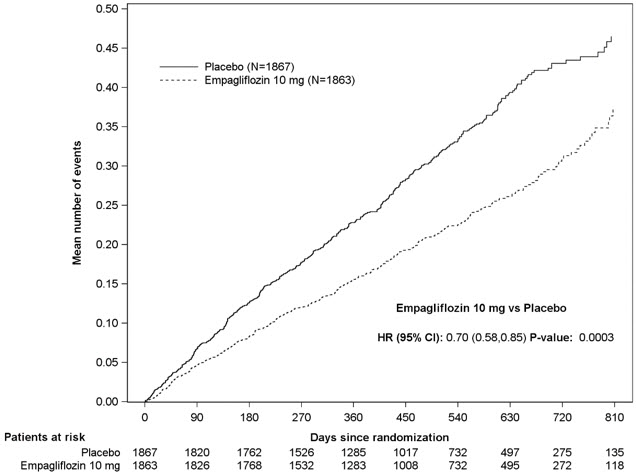
The results of the primary composite were generally consistent across the pre- specified subgroups (see Figure 9).
Figure 9 Treatment Effects for the Primary Composite Endpoint (CV Death and Hospitalization for Heart Failure) Subgroup Analysis (EMPEROR-Reduced)
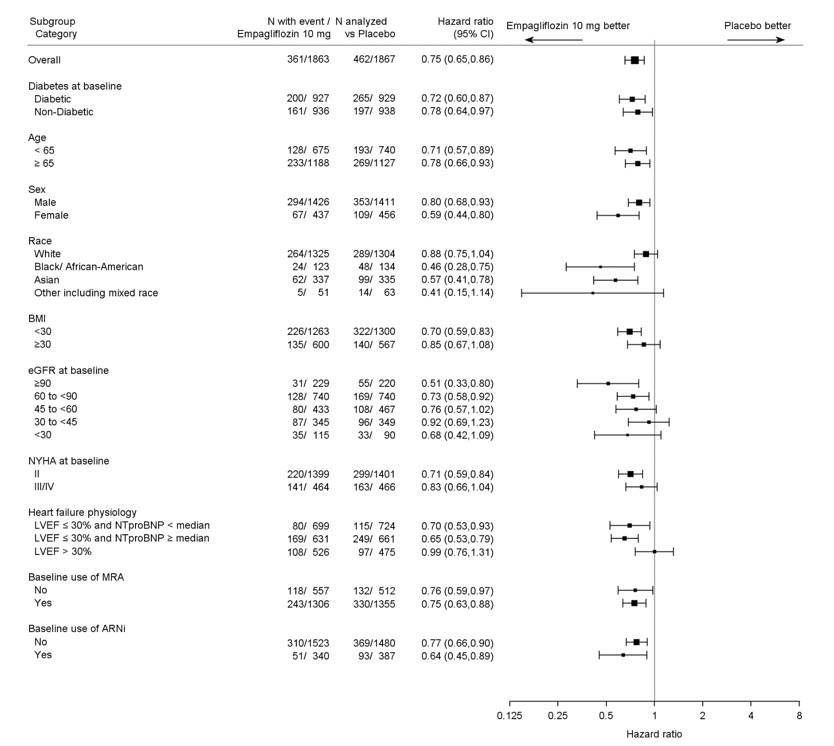
LVEF >30%: Includes both above and below the median NT-proBNP. To be eligible for inclusion, patients with an LVEF >30% were required to meet a higher NT- proBNP threshold than those with LVEF ≤30%, unless they additionally had a history of HHF within the past 12 months.
EMPEROR-Preserved (NCT03057951) was a double-blind trial conducted in patients with chronic heart failure NYHA Class II-IV with LVEF >40% to evaluate the efficacy of JARDIANCE as adjunct to standard of care therapy.
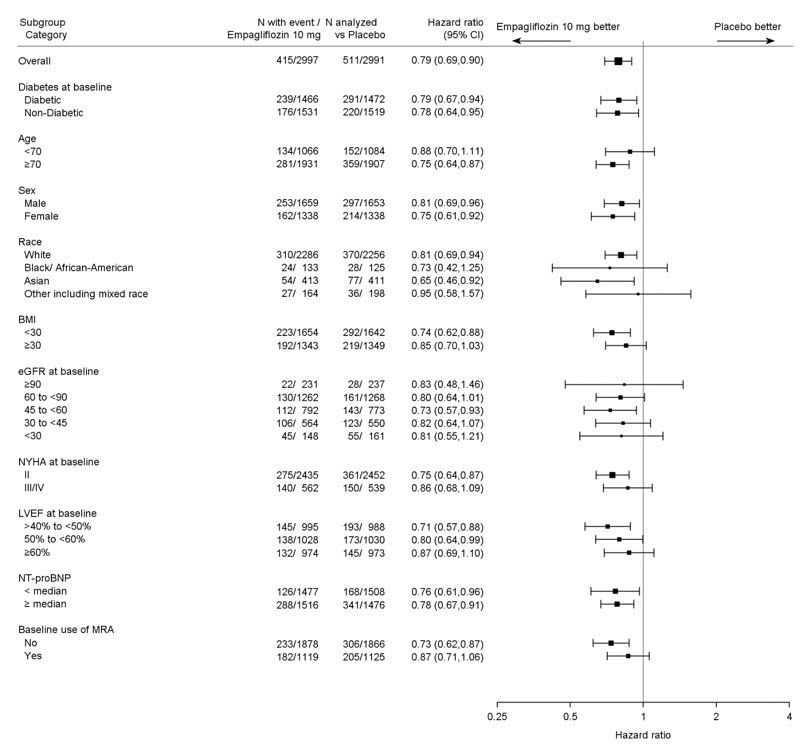
Of 5,988 patients, 2,997 were randomized to JARDIANCE 10 mg and 2,991 to placebo and were followed for a median of 26 months. The mean age of the trial population was 72 years (range: 22 to 100 years) and 55% were men, 45% were women, and 43% were 75 years of age or older. Approximately 76% of the trial population were White, 14% Asian and 4% Black or African American.
At randomization, 82% of patients were NYHA class II, 18% were class III and 0.3% were class IV. The EMPEROR-Preserved trial population included patients with a LVEF <50% (33.1%), with a LVEF 50 to <60% (34.4%) and a LVEF ≥60% (32.5%). At baseline, the mean eGFR was 61 mL/min/1.73 m2 and the median urinary albumin to creatinine ratio (UACR) was 21 mg/g. Approximately half of the patients (50%) had eGFR equal to or above 60 mL/min/1.73 m2, 26% had eGFR 45 to less than 60 mL/min/1.73 m2, 19% had eGFR 30 to less than 45 mL/min/1.73 m2, and 5% had eGFR 20 to less than 30 mL/min/1.73 m2.
At baseline, 81% of patients were treated with ACE inhibitors, ARBs, or ARNI, 86% with beta-blockers, 38% with MRAs, and 86% with diuretics.
The primary endpoint was the time to first event of either CV death or HHF. First and recurrent HHF was assessed as a key secondary endpoint.
JARDIANCE was superior in reducing the risk of the primary composite endpoint compared with placebo, mostly through a reduction in hospitalization for heart failure. JARDIANCE reduced the risk of first and recurrent HHF (see Table 17 and Figures 10 and 11).
Table 17 Treatment Effect for the Primary Composite Endpoint, its Components, and Key Secondary Endpoints|
Placebo |
JARDIANCE 10 mg |
Hazard ratio vs placebo |
p-value | |
|---|---|---|---|---|
|
Number of Patients (%) | ||||
|
aTime to first event | ||||
|
CV death or HHFa |
511 (17.1%) |
415 (13.8%) |
0.79 (0.69, 0.90) |
0.0003 |
|
CV deatha,b |
244 (8.2%) |
219 (7.3%) |
0.91 (0.76, 1.09) | |
|
HHFa |
352 (11.8%) |
259 (8.6%) |
0.71 (0.60, 0.83) | |
|
Number of Events | ||||
|
First and recurrent HHFc |
541 |
407 |
0.73 (0.61, 0.88) |
0.0009 |
Figure 10 Time to First Occurrence of the Primary Composite Endpoint of CV Death or Hospitalization for Heart Failure
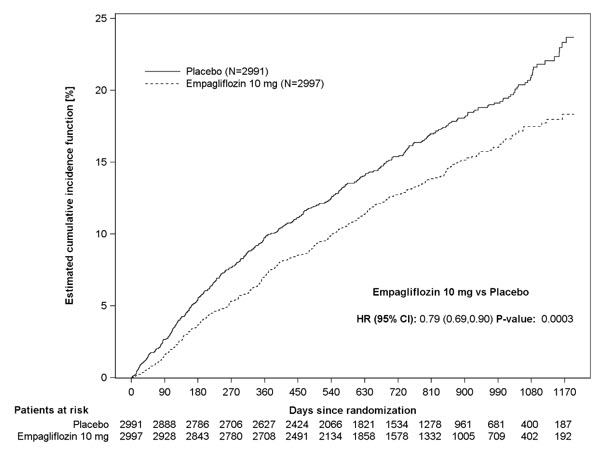
Figure 11 Time to Event of Hospitalization for Heart Failure (First and Recurrent)
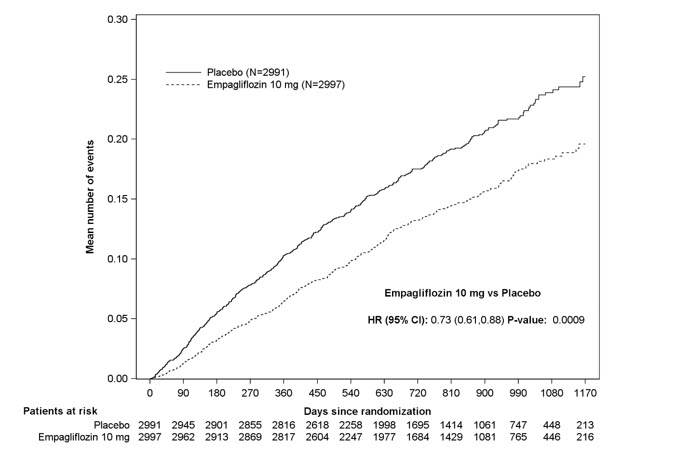
The results of the primary composite endpoint were consistent across the pre- specified subgroups (see Figure 12).
Figure 12 Treatment Effects for the Primary Composite Endpoint (CV Death or Hospitalization for Heart Failure) Subgroup Analysis (EMPEROR-Preserved)