SymlinPen
These highlights do not include all the information needed to use SYMLIN safely and effectively. See full prescribing information for SYMLIN. SYMLIN (pramlintide acetate) injection for subcutaneous useInitial U.S. Approval: 2005
4aea30ff-eb0d-45c1-b114-3127966328ff
HUMAN PRESCRIPTION DRUG LABEL
Dec 18, 2019
AstraZeneca Pharmaceuticals LP
DUNS: 054743190
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
pramlintide acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
pramlintide acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
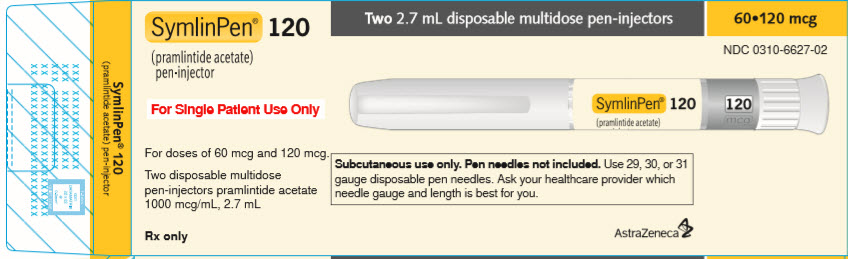
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 2.7 mL pen-injector
SymlinPen**®**** 120**
(pramlintide acetate)
pen-injector
Two 2.7 mL disposable multidose pen-injectors 60•120 mcg NDC 0310-6627-02
For Single Patient Use Only
For doses of 60 mcg and 120 mcg.
Two disposable multidose pen-injectors pramlintide acetate 1000 mcg/mL, 2.7 mL
Subcutaneous use only. Pen needles not included. Use 29, 30, or 31 gauge disposable pen needles. Ask your healthcare provider which needle gauge and length is best for you.
Rx Only AstraZeneca
****
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Insulin
The pharmacokinetic parameters of pramlintide are altered when SYMLIN is mixed in the same syringe with regular, NPH, and 70/30 premixed formulations of recombinant human insulin. SYMLIN and insulin must not be mixed and must be administered as separate injections [see Dosage and Administration (2.4), Warnings and Precautions (5.4), and Clinical Pharmacology (12.3)].
7.2 Oral Medications
SYMLIN has the potential to delay the absorption of concomitantly administered oral medications. When the rapid onset or threshold concentration of a concomitant orally administered medication is a critical determinant of effectiveness (such as with analgesics, antibiotics, and oral contraceptives), the medication should be administered at least 1 hour prior to SYMLIN injection or 2 hours after SYMLIN injection [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
7.3 Drugs Affecting Gastrointestinal Motility
Due to its effects on gastric emptying, SYMLIN should not be considered for patients taking medications that alter gastrointestinal motility (e.g., anticholinergic agents such as atropine) or medications that slow the intestinal absorption of nutrients (e.g., alpha-glucosidase inhibitors). Patients using these medications have not been studied in SYMLIN clinical trials [see Warnings and Precautions (5.6)].
7.4 Drugs Affecting Glucose Metabolism
The following are examples of medications that may increase the susceptibility to hypoglycemia when administered with SYMLIN: anti-diabetic products, angiotensin converting enzyme (ACE) inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, salicylates, somatostatin analogs, and sulfonamide antibiotics. SYMLIN and these drugs should be coadministered with caution.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
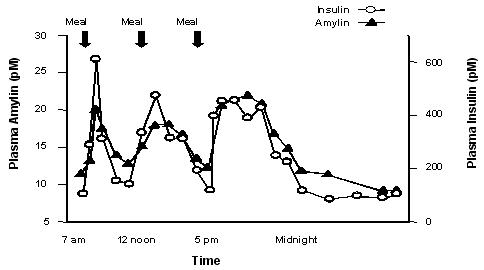
Pramlintide is an analog of human amylin. Amylin is colocated with insulin in secretory granules and cosecreted with insulin by pancreatic beta cells in response to food intake. Amylin and insulin show similar fasting and postprandial patterns in healthy individuals (Figure 1).
|
Figure 1: Secretion Profile of Amylin and Insulin in Healthy Adults |
|
|
In patients with type 1 and type 2 diabetes, there is reduced secretion from pancreatic beta cells of both insulin and amylin in response to food.
Amylin affects the rate of postprandial glucose appearance through a variety of mechanisms, as determined by nonclinical studies. Amylin slows gastric emptying (i.e., the rate at which food is released from the stomach to the small intestine) without altering the overall absorption of nutrients. In addition, amylin suppresses glucagon secretion (not normalized by insulin alone), which leads to suppression of endogenous glucose output from the liver. Amylin also regulates food intake due to centrally-mediated modulation of appetite.
In human studies, pramlintide, acting as an amylin analog, slows gastric emptying, reduces the postprandial rise in plasma glucagon, and modulates satiety leading to decreased caloric intake.
12.2 Pharmacodynamics
In clinical studies in patients with type 1 diabetes and patients with type 2 diabetes using mealtime insulin, SYMLIN reduced mean postprandial glucose concentrations, reduced glucose fluctuations, and reduced food intake.
Reduction in Postprandial Glucose Concentrations
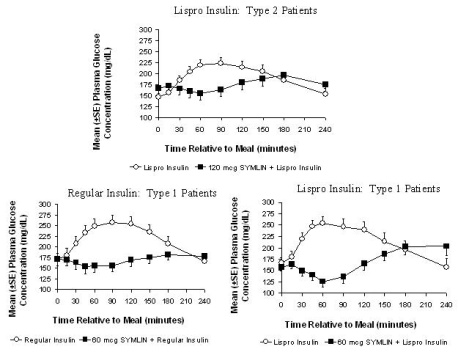
In a randomized, single-blind, placebo-controlled, crossover study, 19 subjects with type 2 diabetes using insulin lispro, 19 subjects with type 1 diabetes using regular human insulin, and 21 subjects with type 1 diabetes using insulin lispro underwent mixed-meal tests. SYMLIN administered subcutaneously immediately prior to a meal reduced plasma glucose concentrations following the meal when used with mealtime insulin (rapid- acting insulin analogs or regular human insulin) (Figure 2). When rapid-acting insulin analogs were used, plasma glucose concentrations tended to rise during the interval between 150 minutes following SYMLIN injection and the next meal [see Dosage and Administration (2)].
|
Figure 2: Postprandial Plasma Glucose Profiles in Patients with Type 1 Diabetes or Type 2 Diabetes Receiving SYMLIN and Insulin Compared to Those Receiving Insulin Alone |
|
|
While SYMLIN reduces postprandial glucose, clinical studies employing a controlled hypoglycemic challenge have demonstrated that SYMLIN does not alter the counter-regulatory hormonal response to insulin-induced hypoglycemia. Likewise, in SYMLIN-treated patients, the perception of hypoglycemic symptoms was not altered with plasma glucose concentrations as low as 45 mg/dL. In a separate clinical trial pramlintide also reduced the 24-hour glucose fluctuations based upon 24-hour glucose monitoring.
Reduced Food Intake
A single, subcutaneous dose of 30 mcg of SYMLIN to patients with type 1 diabetes and 120 mcg of SYMLIN to patients with type 2 diabetes administered 1 hour prior to an unlimited buffet meal was associated with reductions in total caloric intake (placebo-subtracted mean changes of ~21% and 23%, respectively), which occurred without decreases in meal duration.
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of pramlintide following a single subcutaneous dose of SYMLIN is approximately 30% to 40%. Subcutaneous administration of different doses of SYMLIN into the abdominal area or thigh of healthy individuals showed a linear, dose-dependent increase in maximum plasma concentrations (Cmax) and overall exposure (AUC) (Table 5).
Table 5: Mean Pharmacokinetic Parameters Following Administration of Single Subcutaneous Doses of SYMLIN|
Subcutaneous Dose |
AUC**(0-∞)** |
C****max |
T****max |
Elimination t****½ |
|---|---|---|---|---|
|
30 |
3750 |
39 |
21 |
55 |
|
60 |
6778 |
79 |
20 |
49 |
|
90 |
8507 |
102 |
19 |
51 |
|
120 |
11970 |
147 |
21 |
48 |
Injection of SYMLIN into the arm in obese patients with type 1 or type 2 diabetes showed higher overall exposure (20%-36%) with greater variability (% CV for AUC: 73%-106%), compared with exposure after injection of SYMLIN into the abdominal area or thigh.
Relative bioavailability of pramlintide was not significantly different between obese and non-obese patients and based on BMI or skin fold thickness. Injections administered with 6.0-mm and 12.7-mm needles yielded similar bioavailability.
Distribution
SYMLIN does not extensively bind to red blood cells or albumin (approximately 40% of the drug is unbound in plasma).
Metabolism and Elimination
In healthy individuals, the half-life of pramlintide is approximately 48 minutes. The primary metabolite, Des-lys1 pramlintide (2-37 pramlintide), is biologically active in vitro. Overall exposure (AUC) to pramlintide is relatively constant with repeat dosing of SYMLIN, indicating no bioaccumulation.
Specific Populations
Renal Impairment
No studies have been conducted in patients with end-stage renal disease. In a single-dose pharmacokinetic study in patients with type 1 diabetes, 60 mcg of SYMLIN was administered to 4 patients with normal renal function (ClCr >90 mL/min), 9 patients with mild renal impairment (ClCr 60-89 mL/min), 5 patients with moderate renal impairment (ClCr 30-59 mL/min) and 3 patients with severe renal impairment (ClCr 15-29 mL/min). No statistically significant differences were noted in total (AUC0-∞) and peak (Cmax) exposure of pramlintide for mild, moderate, and severe renal impairment categories in comparison to patients with normal renal function; although, inter-patient variability in pharmacokinetic parameters was high.
Hepatic Impairment
Pharmacokinetic studies have not been conducted in patients with hepatic impairment.
Geriatric
Pharmacokinetic studies have not been conducted in the geriatric population [see Use in Specific Populations (8.5)].
Pediatric
The efficacy and safety of SYMLIN have not been established in the pediatric population. The use of SYMLIN is not recommended in pediatric patients due to the risk of severe hypoglycemia [see Warnings and Precautions (5.1, 5.2)].
Gender
No study has been conducted to evaluate the effect of gender on pramlintide pharmacokinetics.
Race/Ethnicity
No study has been conducted to evaluate the effect of ethnicity on pramlintide pharmacokinetics.
Drug Interactions
Effect of Pre-Mixing SYMLIN with Insulin
Pharmacokinetic profiles of pramlintide and insulins after coadministration of 30 mcg SYMLIN with different insulins (regular, NPH, and 70/30 premixed formulations of recombinant human insulin) as one subcutaneous injection, premixed in one syringe, were compared to those observed after the coadministration of SYMLIN and different insulins given as separate subcutaneous injections. The effects of premixing on pramlintide pharmacokinetics varied across the different insulin products with a maximum decrease of 40% in pramlintide Cmax and a maximum increase of 36% in pramlintide AUC0-∞. Similarly, effects of premixing on insulin pharmacokinetics varied across different insulin products with a maximum increase of 15% in insulin Cmax and up to a 20% increase in insulin AUC0-600min. Always administer SYMLIN and insulin as separate injections and never mix [see Warnings and Precautions (5.4)].
Acetaminophen
When 1000 mg acetaminophen was given within 0, 1, and 2 hours after a 120 mcg SYMLIN injection in patients with type 2 diabetes (n=24), acetaminophen Cmax decreased by 29%, 23%, and 20%, respectively compared to placebo. The time to maximum plasma concentration or Tmax increased by 72, 48, and 48 minutes, respectively. SYMLIN did not significantly affect acetaminophen Tmax or Cmax when acetaminophen was administered 1 to 2 hours before SYMLIN injection. SYMLIN did not affect acetaminophen AUC regardless of the time of acetaminophen administration in relation to SYMLIN injection.
Oral Contraceptives
When a single dose of a combination oral contraceptive product, containing 30 mcg ethinyl estradiol and 300 mcg norgestrel, was administered 15 minutes after SYMLIN injection (90 mcg dose) in healthy female subjects, there was no statistically significant change in the Cmax and AUC of ethinyl estradiol. However, the norgestrel Cmax was reduced by about 30% and Tmax was delayed by 45 minutes; there was no effect on norgestrel AUC. The clinical relevance of this change is unknown.
Ampicillin
The effect of concomitant administration of SYMLIN and ampicillin was evaluated in healthy volunteers. The administration of a single oral 500 mg dose of ampicillin 15 minutes after a single dose of SYMLIN (90 mcg) did not alter the Cmax or AUC for ampicillin. However, the Tmax for ampicillin was delayed by approximately 60 minutes.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A two-year carcinogenicity study was conducted in CD-1 mice with doses of 0.2, 0.5, and 1.2 mg/kg/day of pramlintide (32, 67, and 159 times the exposure resulting from the human dose of 360 mcg/day based on area under the plasma concentration curve or AUC, respectively). No drug-induced tumors were observed. A two-year carcinogenicity study was conducted in Sprague-Dawley rats with doses of 0.04, 0.2, and 0.5 mg/kg/day of pramlintide (3, 9, and 25 times the exposure resulting from the human dose of 360 mcg/day based on AUC, respectively). No drug-induced tumors were observed in any organ.
Mutagenesis
Pramlintide was not mutagenic in the Ames test and did not increase chromosomal aberration in the human lymphocytes assay. Pramlintide was not clastogenic in the in vivo mouse micronucleus test or in the chromosomal aberration assay utilizing Chinese hamster ovary cells.
Impairment of Fertility
Administration of 0.3, 1, or 3 mg/kg/day of pramlintide (up to 140 times the human dose of 360 mcg/day based on exposure) prior to and during mating had no significant effects on fertility in male or female rats. The highest dose of 3 mg/kg/day resulted in dystocia in 8/12 female rats secondary to maternal toxicity and significant decreases in serum calcium levels.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
SYMLIN Injection is available in the following package sizes:
•
SymlinPen® 60 pen-injector, containing 1000 mcg/mL pramlintide (as acetate)
Two 1.5 mL disposable multidose pen-injectors
(NDC 0310-6615-02)
•
SymlinPen® 120 pen-injector, containing 1000 mcg/mL pramlintide (as acetate)
Two 2.7 mL disposable multidose pen-injectors
(NDC 0310-6627-02)
Storage and Handling
SYMLIN pen-injectors not in use: Refrigerate (2°C to 8°C; 36°F to 46°F), and protect from light. Do not freeze. Do not use if product has been frozen. Unused SYMLIN (opened or unopened) should not be used after the expiration (EXP) date printed on the carton and the label.
SYMLIN pen-injectors in use: After first use, refrigerate or keep at a temperature not greater than 86°F (30°C) for 30 days. Use within 30 days, whether or not refrigerated.
Storage conditions are summarized in Table 9.
Table 9: Storage Conditions|
Dosage Form |
Unopened (not in use) |
Open (in use) |
|---|---|---|
|
1.5 mL pen-injector |
Until Expiration Date |
Use Within 30 Days |
|
2.7 mL pen-injector |
INSTRUCTIONS FOR USE SECTION
Instructions for Use
SymlinPen® (SĬM-lĭnPεn) 60
(pramlintide acetate)
Pen-Injector
Read the Medication Guide and these Instructions for Use before you start using SYMLIN and each time you get a refill. There may be new information.
Do not share your SymlinPen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Important:
•
Ask your healthcare provider about your dose of SYMLIN and how to inject SYMLIN the right way before you inject it for the first time.
•
Use a new needle for each SYMLIN injection.**Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.**
•
This SymlinPen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of this SymlinPen.
•
Check SYMLIN before you use it. SYMLIN should be clear and colorless.**Do not** use SYMLIN if the liquid looks cloudy or colored or has lumps or particles in it.
•
Your SymlinPen may look empty because SYMLIN is a clear and colorless liquid.
•
Small bubbles will not hurt you or affect your dose of SYMLIN.
•
**Do not** transfer SYMLIN from your SymlinPen into a syringe.
•
**Do not inject your dose of SYMLIN if you**:
∘
have low blood sugar (hypoglycemia)
∘
do not plan to eat.**Do not** inject SYMLIN if you skip a meal. Wait until the next meal and take your usual dose of SYMLIN at that meal.
∘
plan to eat a meal with less than 250 calories or 30 grams of carbohydrate
∘
are sick and cannot eat your usual meal
∘
are having surgery or a medical test where you cannot eat
•
If you take more than your prescribed dose of SYMLIN, you may get nauseous or vomit, and may not be able to eat the amount of food you usually eat. If you take more SYMLIN than your prescribed dose, pay careful attention to the amount of insulin you use because you may be at more risk for low blood sugar. Contact your healthcare provider for guidance.
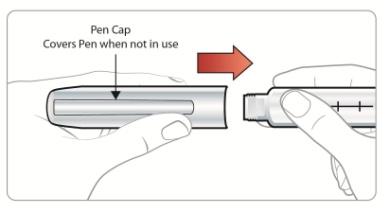
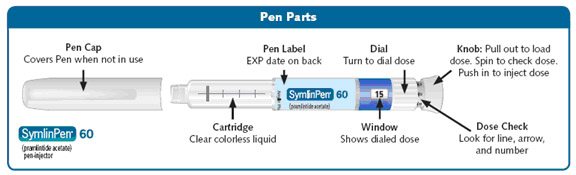
Supplies you will need to give each injection of SYMLIN:
•
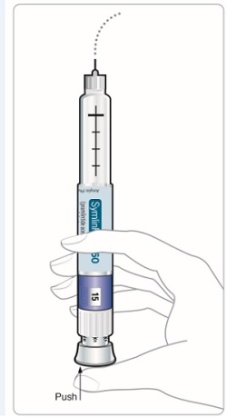
SymlinPen® 60 Pen-Injector (Pen) (See Figure A)
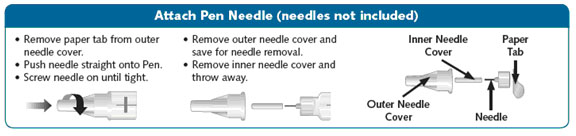
•
a new SymlinPen compatible needle (See Figure B). Pen needles are not included. Use 29, 30, or 31 gauge disposable pen needles. Ask your healthcare provider which needle gauge and length is best for you.
•
alcohol swab
•
1 sharps container for throwing away used SymlinPens and needles.**See “Disposing of used SymlinPens and needles” at the end of these Instructions for Use.**
(Figure A)
(Figure B)
Preparing your SymlinPen:
•
Wash your hands with soap and water.
•
Check the SymlinPen label before each use to make sure you have the right SymlinPen.
•
Check the expiration date (EXP) on the SymlinPen label (See Figure A).**Do not** use the SymlinPen past the expiration date printed on the label.
•
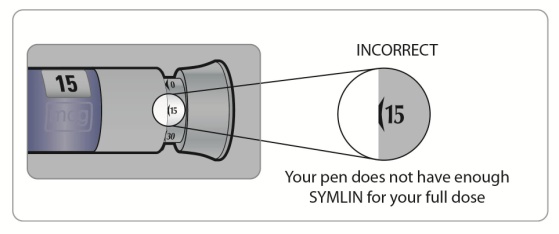
Check the liquid in your SymlinPen to make sure you have enough SYMLIN left in your SymlinPen to load your correct dose.
∘
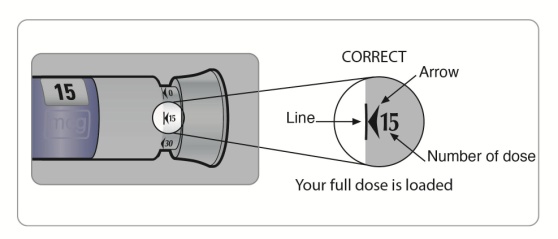
The lines on the cartridge show about how much SYMLIN is left in your SymlinPen. When the top of the plunger is at the thickest line on the cartridge, your SymlinPen is almost empty (See Figure C).
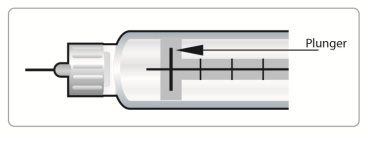
(Figure C)
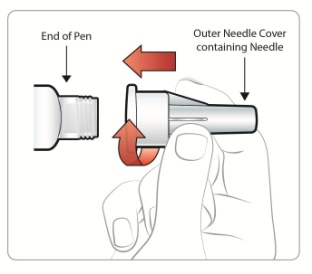
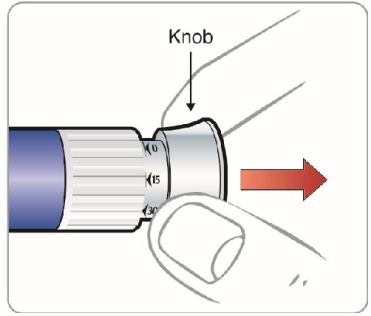
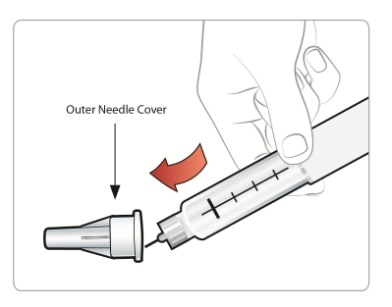
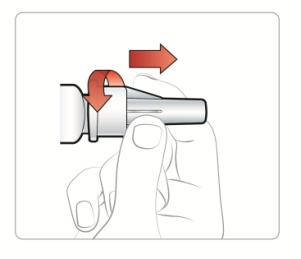
Attaching a Needle:
|
Step 1: • |
Figure D |
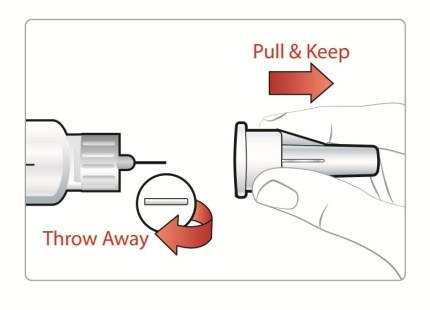
|
Step 2: • • |
Figure E |
|
Step 3: • |
Figure F |
|
Step 4: • • |
Figure G |
New Pen Setup - Priming your SymlinPen:
Note:
•
Steps 5 through 7 are**only** required if you are using your SymlinPen for the first time.**Do not repeat Step 1 through 4 before each dose.**
•
**If you have already primed your SymlinPen, go to Step 8** for instructions about giving your scheduled dose.
|
Step 5: • ∘ |
Figure H |
|
Step 6: • |
Figure I |
|
Step 7: • ∘ ▪ ▪ |
Figure J |
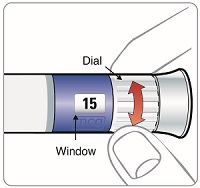
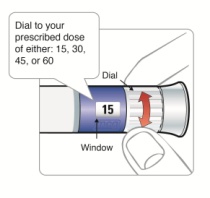
Selecting your routine dose:
|
Step 8: • |
Figure K |
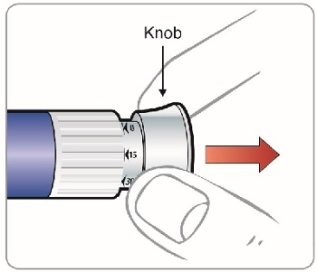
|
Step 9: • |
Figure L |
|
Step 10: • • ∘ ∘ |
Figure M
Figure N |
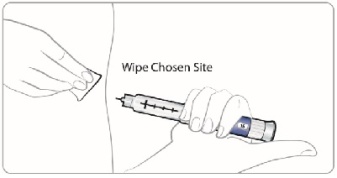
Giving your SYMLIN injection:
•
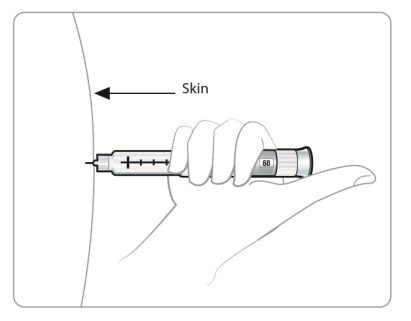
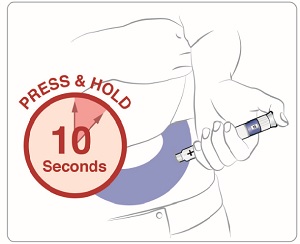
Inject your SYMLIN exactly as your doctor has shown you.
•
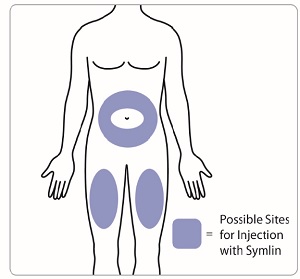
Change (rotate) injection sites for SYMLIN. Inject SYMLIN at a site that is more than 2 inches away from your insulin injection.**Do not** inject SYMLIN and insulin in the same site.
•
To help reduce the chances of getting a reaction at the injection site, let SYMLIN come to room temperature before you inject it.
|
Step 11: • • • |
Figure O |
|
Step 12: • |
Figure P |
|
Step 13: • |
Figure Q |
|
Step 14: • • ∘ ∘ |
Figure R |
|
Step 15: • |
Figure S |
|
Step 16: • • |
Figure T |
|
Step 17: • |
Figure U |
After your injection:
•
Put your used needles and SymlinPen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
∘
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
▪
made of a heavy-duty plastic,
▪
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
▪
upright and stable during use,
▪
leak-resistant, and
▪
properly labeled to warn of hazardous waste inside the container.
∘
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and SymlinPen. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: <http://www.fda.gov/safesharpsdisposal>
∘
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my SymlinPen?
Unused SymlinPens:
•
Keep SYMLIN in the refrigerator between 36°F to 46°F (2°C to 8°C), until you are ready to use it.
•
**Do not** freeze.**Do not** use SYMLIN if it has been frozen.
•
Keep unopened SYMLIN out of the light.
Used SymlinPens:
•
Store SymlinPens in the refrigerator or at room temperature between 36°F to 86°F (2°C to 30°C) for up to 30 days.
•
Throw away used SymlinPens after 30 days of use, even if a used SymlinPen still has medicine in it.
•
**Do not** use SymlinPens (opened or unopened) after the expiration (EXP) date printed on the carton and the label.
General information about the safe and effective use of SymlinPens.
•
If you drop your SymlinPen, you should prime it before you use it to make sure your SymlinPen works.
•
**Do not** use your SymlinPen if any part looks broken or damaged.
•
**Keep SYMLIN and all medicines out of the reach of children.**
•
It is not known if SYMLIN is safe and effective in children.
If you are having problems using your SymlinPen, go to www.SYMLIN.com or call Information Support at 1-800-236-9933.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
SYMLIN and SymlinPen are registered trademarks of the AstraZeneca group of companies.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
Revised: March 2015