PredniSONE Tablets, USP, 20 mg
PredniSONE Tablets USP, 20 mg
6b360c1f-c862-47ee-a40e-3903c2d6162f
HUMAN PRESCRIPTION DRUG LABEL
Jun 23, 2025
PD-Rx Pharmaceuticals, Inc.
DUNS: 156893695
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
PredniSONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
PredniSONE Tablets, USP 20 mg
Rx Only

DESCRIPTION SECTION
DESCRIPTION
Prednisone is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Prednisone, USP is a white to partially white, crystalline powder. It is very slightly soluble in water; slightly soluble in alcohol, chloroform, dioxane, and methanol.
The chemical name for prednisone is 17,21-dihydroxypregna-1,4-dienne-3,11,20-trione. The structural formula is represented below:
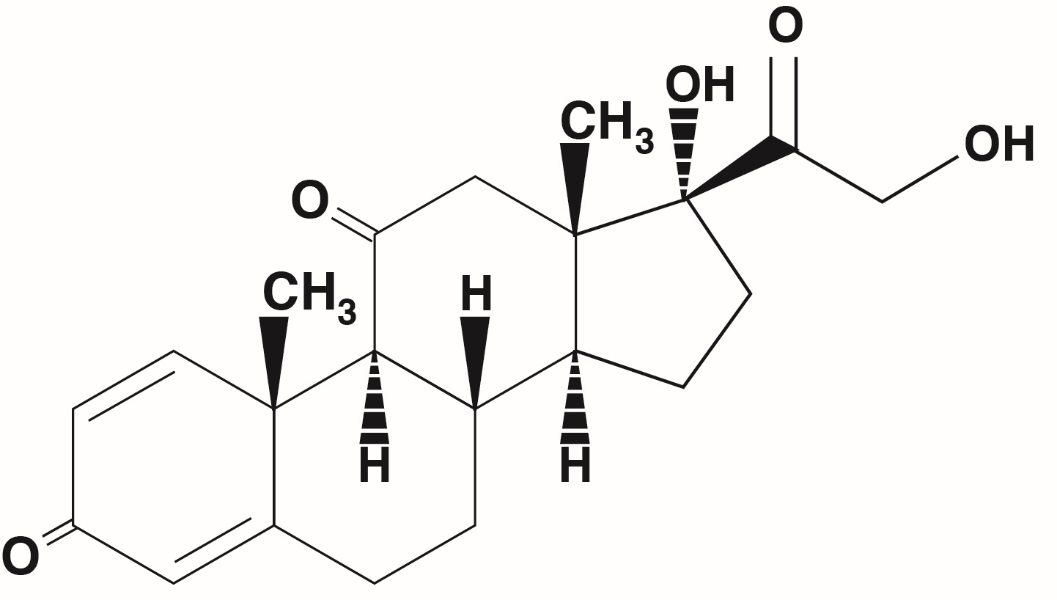
C 21H 26O 5M.W. 358.44
Each tablet, for oral administration, contains 5, 10, or 20 mg of prednisone.
Inactive Ingredients:
PredniSONE Tablets, USP contain the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, colloidal silicon dioxide and talc.
Meets USP Dissolution Test 2.
HOW SUPPLIED SECTION
HOW SUPPLIED
PredniSONE Tablets USP, 20 mg
20 mg – White, round, scored tablets, debossed "Є 173" on one side, plain and scored on the other side.
NDC 72789-473-05: Bottle of 5 Tablets
NDC 72789-473-10: Bottle of 10 Tablets
NDC 72789-473-20: Bottle of 20 Tablets
NDC 72789-473-21: Bottle of 21 Tablets
NDC 72789-473-25: Bottle of 25 Tablets
NDC 72789-473-30: Bottle of 30 Tablets
NDC 72789-473-01: Bottle of 100 Tablets
NDC 72789-473-95: Bottle of 1000 Tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Dispense in a tight, child-resistant container as defined in the USP/NF.
PROTECT FROM MOISTURE.
