Qulipta
These highlights do not include all the information needed to use QULIPTA safely and effectively. See full prescribing information for QULIPTA . QULIPTA (atogepant) tablets, for oral use Initial U.S. Approval: 2021
8c8ab8f4-32bd-497a-befa-70c8a51d8d52
HUMAN PRESCRIPTION DRUG LABEL
Jun 1, 2023
AbbVie Inc.
DUNS: 078458370
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Atogepant
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Atogepant
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Atogepant
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0074-7094-30
Rx Only
QULIPTA**®**
(atogepant) tablets
60 mg
Contains 30 Tablets

INDICATIONS & USAGE SECTION
1****INDICATIONS AND USAGE
QULIPTA is indicated for the preventive treatment of migraine in adults.
QULIPTA is a calcitonin gene-related peptide receptor antagonist indicated for the preventive treatment of migraine in adults. (1)
CONTRAINDICATIONS SECTION
4****CONTRAINDICATIONS
QULIPTA is contraindicated in patients with a history of hypersensitivity to atogepant or any of the components of QULIPTA. Reactions have included anaphylaxis and dyspnea [see Warnings and Precautions (5.1)].
Patients with a history of hypersensitivity to atogepant or to any of the components of QULIPTA. (4)
WARNINGS AND PRECAUTIONS SECTION
5** WARNINGS AND PRECAUTIONS**
5.1** Hypersensitivity Reactions**
Hypersensitivity reactions, including anaphylaxis, dyspnea, rash, pruritus, urticaria, and facial edema, have been reported with use of QULIPTA [see Adverse Reactions (6.2)]. Hypersensitivity reactions can occur days after administration. If a hypersensitivity reaction occurs, discontinue QULIPTA and institute appropriate therapy [see Contraindications (4)].
If a hypersensitivity reaction occurs, discontinue QULIPTA and initiate appropriate therapy. Severe hypersensitivity reactions have included anaphylaxis and dyspnea. These reactions can occur days after administration. (5.1)
ADVERSE REACTIONS SECTION
6** ADVERSE REACTIONS**
6.1** ClinicalTrialsExperience**
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of QULIPTA was evaluated in 2657 patients with migraine who received at least one dose of QULIPTA. Of these, 1225 patients were exposed to QULIPTA for at least 6 months, and 826 patients were exposed for 12 months.
In the 12-week, placebo-controlled clinical studies (Studies 1, 2, and 3), 314 patients received at least one dose of QULIPTA 10 mg once daily, 411 patients received at least one dose of QULIPTA 30 mg once daily, 678 patients received at least one dose of QULIPTA 60 mg once daily, and 663 patients received placebo [see Clinical Studies (14)]. Approximately 88% were female, 75% were White, 13% were Black, 10% were Asian, and 10% were of Hispanic or Latino ethnicity. The mean age at study entry was 41 years (range 18 to 74 years).
The most common adverse reactions (incidence at least 4% and greater than placebo) are nausea, constipation, and fatigue/somnolence.
Table 2 summarizes the adverse reactions that occurred during Studies 1, 2, and 3.
Table 2: Adverse Reactions Occurring with an Incidence of At Least 2% for QULIPTA and Greater than Placebo in Studies 1, 2, and 3*|
Placebo |
QULIPTA**** |
QULIPTA**** |
QULIPTA**** | |
|
Nausea |
3 |
5 |
6 |
9 |
|
Constipation |
2 |
6 |
6 |
8 |
|
Fatigue/Somnolence |
4 |
4 |
4 |
5 |
|
Decreased Appetite |
<1 |
2 |
1 |
3 |
|
Dizziness |
2 |
2 |
2 |
3 |
- 10 mg and 30 mg incidence from Studies 1 and 2; 60 mg pooled incidence from Studies 1, 2, and 3.
The adverse reactions that most commonly led to discontinuation of QULIPTA in these studies were nausea (0.6%), constipation (0.5%), and fatigue/somnolence (0.2%).
Liver Enzyme Elevations
In Study 1, Study 2, and Study 3, the rate of transaminase elevations over 3 times the upper limit of normal was similar between patients treated with QULIPTA (0.9%) and those treated with placebo (1.2%). However, there were cases with transaminase elevations over 3 times the upper limit of normal that were temporally associated with QULIPTA treatment; these were asymptomatic and resolved within 8 weeks of discontinuation. There were no cases of severe liver injury or jaundice.
Decreases in Body Weight
In Study 1, Study 2, and Study 3, the proportion of patients with a weight decrease of at least 7% at any point was 2.5% for placebo, 3.8% for QULIPTA 10 mg, 3.2% for QULIPTA 30 mg, and 5.3% for QULIPTA 60 mg.
6.2** Postmarketing Experience**
The following adverse reactions have been identified during post approval use of QULIPTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity (e.g., anaphylaxis, dyspnea, rash, pruritus, urticaria, facial edema) [see Contraindications (4) and Warnings and Precautions (5.1)]
The most common adverse reactions (at least 4% and greater than placebo) are nausea, constipation, and fatigue/somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7** DRUG INTERACTIONS**
7.1****CYP3A4 Inhibitors
Coadministration of QULIPTA with itraconazole, a strong CYP3A4 inhibitor, resulted in a significant increase in exposure of atogepant in healthy subjects [see Clinical Pharmacology (12.3)]. For episodic migraine, the recommended dosage of QULIPTA with concomitant use of strong CYP3A4 inhibitors is 10 mg once daily. For chronic migraine, avoid concomitant use of strong CYP3A4 inhibitors with QULIPTA [see Dosage and Administration (2.2)]. No dosage adjustment of QULIPTA is needed with concomitant use of moderate or weak CYP3A4 inhibitors.
7.2****CYP3A4 Inducers
Coadministration of QULIPTA with steady state rifampin, a strong CYP3A4 inducer, resulted in a significant decrease in exposure of atogepant in healthy subjects [see Clinical Pharmacology (12.3)]. Concomitant administration of QULIPTA with moderate inducers of CYP3A4 can also result in decreased exposure of atogepant. Coadministration of QULIPTA with steady-state topiramate, a weak CYP3A4 inducer, resulted in decreased exposure of atogepant in healthy subjects [see Clinical Pharmacology (12.3)].
For episodic migraine, the recommended dosage of QULIPTA with concomitant use of strong, moderate, or weak CYP3A4 inducers is 30 mg or 60 mg once daily [see Dosage and Administration (2.2)].
For chronic migraine, avoid concomitant use of strong, moderate, or weak CYP3A4 inducers with QULIPTA [see Dosage and Administration (2.2)].
7**.3****OATP Inhibitors**
Coadministration of QULIPTA with single dose rifampin, an OATP inhibitor, resulted in a significant increase in exposure of atogepant in healthy subjects [see Clinical Pharmacology (12.3)]. For episodic migraine, the recommended dosage of QULIPTA with concomitant use of OATP inhibitors is 10 mg or 30 mg once daily. For chronic migraine, the recommended dosage of QULIPTA with concomitant use of OATP inhibitors is 30 mg once daily [see Dosage and Administration (2.2)].
Recommended dosage modifications:
-
Strong CYP3A4 Inhibitor (2.2, 7.1):
○ Episodic migraine: 10 mg once daily.
○ Chronic migraine: avoid use. -
Strong, Moderate, or Weak CYP3A4 Inducers (2.2, 7.2):
○ Episodic migraine: 30 mg or 60 mg once daily.
○ Chronic migraine: avoid use. -
OATP Inhibitors (2.2, 7.3):
○ Episodic migraine: 10 mg or 30 mg once daily.
○ Chronic migraine: 30 mg once daily.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage (1) |
4/2023 |
|
Dosage and Administration (2.1, 2.2) |
4/2023 |
|
Contraindications (4) |
4/2023 |
|
Warnings and Precautions (5.1) |
4/2023 |
DOSAGE FORMS & STRENGTHS SECTION
3****DOSAGE FORMS AND STRENGTHS
QULIPTA 10 mg is supplied as white to off-white, round biconvex tablets debossed with “A” and “10” on one side.
QULIPTA 30 mg is supplied as white to off-white, oval biconvex tablets debossed with “A30” on one side.
QULIPTA 60 mg is supplied as white to off-white, oval biconvex tablets debossed with “A60” on one side.
Tablets: 10 mg, 30 mg, and 60 mg. (3)
SPL PATIENT PACKAGE INSERT SECTION
|
PATIENT INFORMATION | |
|
What isQULIPTA?
It is not known if QULIPTA is safe and effective in children. | |
|
Do not take QULIPTA if you:
| |
|
Before you takeQULIPTA tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. QULIPTA may affect the way other medicines work, and other medicines may affect how QULIPTA works. Your healthcare provider may need to change the dose of QULIPTA when taken with certain other medicines. | |
|
Keep a list of medicines you take to show to your healthcare provider or pharmacist when you get a new medicine. | |
|
How should I takeQULIPTA?
| |
|
What are the possible side effects ofQULIPTA? | |
|
|
|
|
| |
|
The most common side effects of****QULIPTA****include**:** nausea,
constipation, and fatigue/sleepiness. | |
|
How should I storeQULIPTA?
KeepQULIPTA and all medicines out of the reach of children. | |
|
General information about the safe and effective use ofQULIPTA. | |
|
What are the ingredients inQULIPTA? Manufactured for: |
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 6/2023
20078877
DOSAGE & ADMINISTRATION SECTION
2****DOSAGE AND ADMINISTRATION
2.1** Recommended Dosage**
QULIPTA is taken orally with or without food.
Episodic Migraine
The recommended dosage of QULIPTA for episodic migraine is 10 mg, 30 mg, or 60 mg taken once daily.
Chronic Migraine
The recommended dosage of QULIPTA for chronic migraine is 60 mg taken once daily.
2.2** DosageModifications******
Dosing modifications for concomitant use of specific drugs and for patients with renal impairment are provided in Table 1.
Table 1: Dosage Modifications for Drug Interactions and for Specific Populations|
Dosage Modifications |
Recommended****Once Daily |
Usage and |
|
Concomitant Drug**[see Drug Interactions (7)]** | ||
|
Strong CYP3A4 Inhibitors (7.1) |
10 mg |
Avoid use |
|
Strong, Moderate, or Weak CYP3A4 Inducers (7.2) |
30 mg or 60 mg |
Avoid use |
|
OATP Inhibitors (7.3) |
10 mg or 30 mg |
30 mg |
|
Renal Impairment**[see Use in Specific Populations******(8)] | ||
|
Severe Renal Impairment and End-Stage Renal Disease (CLcr <30 mL/min) (8.6) |
10 mg |
Avoid use |
-
QULIPTA is taken orally with or without food. (2.1)
-
For episodic migraine, the recommended dosage is 10 mg, 30 mg, or 60 mg taken once daily. (2.1)
-
For chronic migraine, the recommended dosage is 60 mg taken once daily. (2.1)
-
Severe Renal Impairment or End-Stage Renal Disease (2.2, 8.6):
-
Episodic migraine: 10 mg once daily.
-
Chronic migraine: Avoid use.
-
USE IN SPECIFIC POPULATIONS SECTION
8** USE IN SPECIFIC POPULATIONS**
8.1** Pregnancy**
Risk Summary
There are no adequate data on the developmental risk associated with the use of QULIPTA in pregnant women. In animal studies, oral administration of atogepant during the period of organogenesis (rats and rabbits) or throughout pregnancy and lactation (rats) resulted in adverse developmental effects (decreased fetal and offspring body weight in rats; increased incidence of fetal structural variations in rabbits) at exposures greater than those used clinically [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The estimated rate of major birth defects (2.2%-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Data
Animal Data
Oral administration of atogepant (0, 5, 15, 125, or 750 mg/kg/day) to pregnant rats during the period of organogenesis resulted in decreases in fetal body weight and in skeletal ossification at the two highest doses tested (125 and 750 mg/kg), which were not associated with maternal toxicity. At the no-effect dose (15 mg/kg/day) for adverse effects on embryofetal development, plasma exposure (AUC) was approximately 4 times that in humans at the maximum recommended human dose (MRHD) of 60 mg/day.
Oral administration of atogepant (0, 30, 90, or 130 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in an increase in fetal visceral and skeletal variations at the highest dose tested (130 mg/kg/day), which was associated with minimal maternal toxicity. At the no-effect dose (90 mg/kg/day) for adverse effects on embryofetal development, plasma exposure (AUC) was approximately 3 times that in humans at the MRHD.
Oral administration of atogepant (0, 15, 45, or 125 mg/kg/day) to rats throughout gestation and lactation resulted in decreased pup body weight at the highest dose tested (125 mg/kg/day), which persisted into adulthood. At the no-effect dose (45 mg/kg/day) for adverse effects on pre- and postnatal development, plasma exposure (AUC) was approximately 5 times that in humans at the MRHD.
8.2****Lactation
There are no data on the presence of atogepant in human milk, the effects of atogepant on the breastfed infant, or the effects of atogepant on milk production. In lactating rats, oral dosing with atogepant resulted in levels of atogepant in milk approximately 2-fold higher than that in maternal plasma. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for QULIPTA and any potential adverse effects on the breastfed infant from QULIPTA or from the underlying maternal condition.
8.4** Pediatric Use**
Safety and effectiveness in pediatric patients have not been established.
8.5** Geriatric Use**
Population pharmacokinetic modeling suggests no clinically significant pharmacokinetic differences between elderly and younger subjects. Clinical studies of QULIPTA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
The renal route of elimination plays a minor role in the clearance of atogepant [see Clinical Pharmacology (12.3)]. For episodic migraine, in patients with severe renal impairment (CLcr 15-29 mL/min) and in patients with end-stage renal disease (ESRD) (CLcr <15 mL/min), the recommended dosage of QULIPTA is 10 mg once daily; in patients with ESRD undergoing intermittent dialysis, QULIPTA should preferably be taken after dialysis [see Dosage and Administration (2.2)]. For chronic migraine, avoid use of QULIPTA in patients with severe renal impairment and in patients with ESRD. No dose adjustment is recommended for patients with mild or moderate renal impairment.
8.7Hepatic Impairment
No dose adjustment of QULIPTA is recommended for patients with mild or moderate hepatic impairment. Avoid use of QULIPTA in patients with severe hepatic impairment [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
-
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
-
Avoid use in patients with severe hepatic impairment. (8.7)
DESCRIPTION SECTION
11****DESCRIPTION
The active ingredient of QULIPTA is atogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of atogepant is (3'S)-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide, and it has the following structural formula:
![The active ingredient of TRADENAME is atogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of atogepant is (S)-N-((3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide
and has the following structural
formula:](https://medical-cdn.nocode.com/data-engine/drug_fda/8c8ab8f4-32bd-497a-befa-70c8a51d8d52/qulipta_01_jpg/9a924382387a746b5eab1b33583560b4.jpg)
The molecular formula is C29H23F6N5O3 and molecular weight is 603.5. Atogepant is a white to off-white powder. It is freely soluble in ethanol, soluble in methanol, sparingly soluble in acetone, slightly soluble in acetonitrile, and practically insoluble in water.
QULIPTA is available as tablets for oral administration containing 10 mg, 30 mg, or 60 mg atogepant. The inactive ingredients include colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, polyvinylpyrrolidone vinyl acetate copolymer, sodium chloride, sodium stearyl fumarate, and vitamin E polyethylene glycol succinate.
CLINICAL PHARMACOLOGY SECTION
12****CLINICAL PHARMACOLOGY
12.1****Mechanism of Action
Atogepant is a calcitonin gene-related peptide (CGRP) receptor antagonist.
12.2** Pharmacodynamics**
Cardiac Electrophysiology
At a dose 5 times the maximum recommended daily dose, QULIPTA does not prolong the QT interval to any clinically relevant extent.
12.3****Pharmacokinetics
Absorption
Following oral administration of QULIPTA, atogepant is absorbed with peak plasma concentrations at approximately 1 to 2 hours. Atogepant displays dose- proportional pharmacokinetics up to 170 mg per day (approximately 3 times the highest recommended dosage), with no accumulation.
Effect of Food
When QULIPTA was administered with a high-fat meal, the food effect was not significant (AUC and Cmax were reduced by approximately 18% and 22%, respectively, with no effect on median time to maximum atogepant plasma concentration). QULIPTA was administered without regard to food in clinical efficacy studies.
Distribution
Plasma protein binding of atogepant was not concentration-dependent in the range of 0.1 to 10 µM; the unbound fraction of atogepant was approximately 4.7% in human plasma. The mean apparent volume of distribution of atogepant (Vz/F) after oral administration is approximately 292 L.
Elimination
Metabolism
Atogepant is eliminated mainly through metabolism, primarily by CYP3A4. The parent compound (atogepant), and a glucuronide conjugate metabolite (M23) were the most prevalent circulating components in human plasma.
Excretion
The elimination half-life of atogepant is approximately 11 hours. The mean apparent oral clearance (CL/F) of atogepant is approximately 19 L/hr. Following single oral dose of 50 mg 14C-atogepant to healthy male subjects, 42% and 5% of the dose was recovered as unchanged atogepant in feces and urine, respectively.
Specific Populations
Patients with Renal Impairment
The renal route of elimination plays a minor role in the clearance of atogepant. Based on a population pharmacokinetic analysis, there is no significant difference in the pharmacokinetics of atogepant in patients with mild or moderate renal impairment (CLcr 30-89 mL/min) relative to those with normal renal function (CLcr >90 mL/min). Patients with severe renal impairment or end-stage renal disease (ESRD; CLcr <30 mL/min) have not been studied [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
In patients with pre-existing mild (Child-Pugh Class A), moderate (Child-Pugh Class B), or severe (Child-Pugh Class C) hepatic impairment, the total atogepant exposure was increased by 24%, 15%, and 38%, respectively. Due to a potential for liver injury in patients with severe hepatic impairment, avoid use of QULIPTA in patients with severe hepatic impairment [see Use in Specific Populations (8.7)].
Other Specific Populations
Based on a population pharmacokinetic analysis, age, sex, race, and body weight did not have a significant effect on the pharmacokinetics (Cmax and AUC) of atogepant. Therefore, no dose adjustments are warranted based on these factors.
Drug Interactions
In Vitro Studies
Enzymes
In vitro, atogepant is not an inhibitor for CYPs 3A4, 1A2, 2B6, 2C8, 2C9, 2C19, or 2D6 at clinically relevant concentrations. Atogepant does not inhibit MAO-A or UGT1A1 at clinically relevant concentrations. Atogepant is not anticipated to be a clinically significant perpetrator of drug-drug interactions through CYP450s, MAO-A, or UGT1A1 inhibition.
Atogepant is not an inducer of CYP1A2, CYP2B6, or CYP3A4 at clinically relevant concentrations.
Transporters
Atogepant is a substrate of P-gp, BCRP, OATP1B1, OATP1B3, and OAT1. Dose adjustment for concomitant use of QULIPTA with inhibitors of OATP is recommended based on a clinical interaction study with a OATP inhibitor [see Dosage and Administration (2.2)].
Coadministration of atogepant with BCRP and/or P-gp inhibitors is not expected to increase the exposure of atogepant. Atogepant is not a substrate of OAT3, OCT2, or MATE1.
Atogepant is not an inhibitor of P-gp, BCRP, OAT1, OAT3, NTCP, BSEP, MRP3, or MRP4 at clinically relevant concentrations. Atogepant is a weak inhibitor of OATP1B1, OATP1B3, OCT1, and MATE1. No clinical drug interactions are expected for atogepant as a perpetrator with these transporters.
In Vivo Studies
CYP3A4 Inhibitors
Co-administration of QULIPTA with itraconazole, a strong CYP3A4 inhibitor, resulted in a clinically significant increase (Cmax by 2.15-fold and AUC by 5.5-fold) in the exposure of atogepant in healthy subjects [see Drug Interactions (7.1)].
Physiologically based pharmacokinetic (PBPK) modeling suggested co- administration of QULIPTA with moderate or weak CYP3A4 inhibitors increase atogepant AUC by 1.7- and 1.1-fold, respectively. The changes in atogepant exposure when coadministered with weak or moderate CYP3A4 inhibitors are not expected to be clinically significant.
CYP3A4 Inducers
Co-administration of QULIPTA with rifampin, a strong CYP3A4 inducer, decreased atogepant AUC by 60% and Cmax by 30% in healthy subjects [see Drug Interactions (7.2)]. No dedicated drug interaction studies were conducted to assess concomitant use with moderate CYP3A4 inducers. Moderate inducers of CYP3A4 can decrease atogepant exposure [see Drug Interactions (7.2)]. Co- administration of QULIPTA with topiramate, a weak inducer of CYP3A4, decreased atogepant mean steady-state AUC 0-τ by 25% and mean steady-state Cmax by 24% in healthy subjects [see Drug Interactions (7.2)].
BCRP/OATP/P-gp Inhibitors
Co-administration of QULIPTA with single dose rifampin, an OATP inhibitor, increased atogepant AUC by 2.85-fold and Cmax by 2.23-fold in healthy subjects [see Drug Interactions (7.3)].
Co-administration of QULIPTA with quinidine, a P-gp inhibitor, increased atogepant AUC by 26% and Cmax by 4% in healthy subjects. The changes in atogepant exposure when co-administered with P-gp inhibitors are not expected to be clinically significant.
PBPK modeling suggests that co-administration of QULIPTA with BCRP inhibitors increases atogepant exposure by 1.2-fold. This increase is not expected to be clinically significant.
Other Drug Interaction Evaluations
Co-administration of QULIPTA with oral contraceptive components ethinyl estradiol and levonorgestrel, famotidine, esomeprazole, acetaminophen, naproxen, sumatriptan, or ubrogepant did not result in significant pharmacokinetic interactions for either atogepant or co-administered drugs. Co-administration of QULIPTA with topiramate did not result in clinically significant changes in the pharmacokinetics of topiramate.
NONCLINICAL TOXICOLOGY SECTION
13** NONCLINICAL TOXICOLOGY**
13.1** Carcinogenesis, Mutagenesis, Impairment of Fertility**
Carcinogenicity
Atogepant was administered orally to mice (0, 5, 20, or 75 mg/kg/day in males; 0, 5, 30, 160 mg/kg/day in females) and rats (0, 10, 20, or 100 mg/kg in males; 0, 25, 65, or 200 mg/kg in females) for up to 2 years. There was no evidence of drug-related tumors in either species. Plasma exposures at the highest doses tested in mice and rats were approximately 8 and 20-35 times, respectively, that in humans at the maximum recommended human dose (MRHD) of 60 mg/day.
Mutagenicity
Atogepant was negative in in vitro (Ames, chromosomal aberration test in Chinese Hamster Ovary cells) and in vivo (rat bone marrow micronucleus) assays.
Impairment of Fertility
Oral administration of atogepant (0, 5, 20, or 125 mg/kg/day) to male and female rats prior to and during mating and continuing in females to Gestation Day 7 resulted in no adverse effects on fertility or reproductive performance. Plasma exposures (AUC) at the highest dose tested are approximately 15 times that in humans at the MRHD.
CLINICAL STUDIES SECTION
14** CLINICAL STUDIES**
14.1** Episodic Migraine**
The efficacy of QULIPTA for the preventive treatment of episodic migraine in adults was demonstrated in two randomized, multicenter, double-blind, placebo- controlled studies (Study 1 and Study 2). The studies enrolled patients with at least a 1-year history of migraine with or without aura, according to the International Classification of Headache Disorders (ICHD-3) diagnostic criteria.
In Study 1 (NCT03777059), 910 patients were randomized 1:1:1:1 to receive QULIPTA 10 mg (N = 222), QULIPTA 30 mg (N = 230), QULIPTA 60 mg (N = 235), or placebo (N = 223), once daily for 12 weeks. In Study 2 (NCT02848326), 652 patients were randomized 1:2:2:2 to receive QULIPTA 10 mg (N = 94), QULIPTA 30 mg (N = 185), QULIPTA 60 mg (N = 187), or placebo (N = 186), once daily for 12 weeks. In both studies, patients were allowed to use acute headache treatments (i.e., triptans, ergotamine derivatives, NSAIDs, acetaminophen, and opioids) as needed. The use of a concomitant medication that acts on the CGRP pathway was not permitted for either acute or preventive treatment of migraine. The studies excluded patients with myocardial infarction, stroke, or transient ischemic attacks within six months prior to screening.
Study 1
The primary efficacy endpoint was the change from baseline in mean monthly migraine days (MMD) across the 12-week treatment period. Secondary endpoints included the change from baseline in mean monthly headache days, the change from baseline in mean monthly acute medication use days, the proportion of patients achieving at least a 50% reduction from baseline in mean MMD (3-month average), the change from baseline in mean monthly Activity Impairment in Migraine-Diary (AIM-D) Performance of Daily Activities (PDA) domain scores, the change from baseline in mean monthly AIM-D Physical Impairment (PI) domain scores, across the 12-week treatment period, and the change from baseline at Week 12 for Migraine Specific Quality of Life Questionnaire version 2.1 (MSQ v2.1) Role Function-Restrictive (RFR) domain scores.
The AIM-D evaluates difficulty with performance of daily activities (PDA domain) and physical impairment (PI domain) due to migraine, with scores ranging from 0 to 100. Higher scores indicate greater impact of migraine, and reductions from baseline indicate improvement. The MSQ v2.1 Role Function- Restrictive (RFR) domain score assesses how often migraine impacts function related to daily social and work-related activities over the past 4 weeks, with scores ranging from 0 to 100. Higher scores indicate lesser impact of migraine on daily activities, and increases from baseline indicate improvement.
Patients had a mean age of 42 years (range 18 to 73 years), 89% were female, 83% were White, 14% were Black, and 9% were of Hispanic or Latino ethnicity. The mean migraine frequency at baseline was approximately 8 migraine days per month and was similar across treatment groups. A total of 805 (88%) patients completed the 12-week double-blind study period. Key efficacy results of Study 1 are summarized in Table 3.
Table 3: Efficacy Endpoints in Study 1|
QULIPTA |
QULIPTA |
QULIPTA |
Placebo | |
|
Monthly Migraine Days** (MMD) across 12 weeks** | ||||
|
Baseline |
7.5 |
7.9 |
7.8 |
7.5 |
|
Mean change from baseline |
-3.7 |
-3.9 |
-4.2 |
-2.5 |
|
Difference from placebo |
-1.2 |
-1.4 |
-1.7 | |
|
p-value |
<0.001 |
<0.001 |
<0.001 | |
|
Monthly Headache Days** across 12 weeks** | ||||
|
Baseline |
8.4 |
8.8 |
9.0 |
8.4 |
|
Mean change from baseline |
-3.9 |
-4.0 |
-4.2 |
-2.5 |
|
Difference from placebo |
-1.4 |
-1.5 |
-1.7 | |
|
p-value |
<0.001 |
<0.001 |
<0.001 | |
|
Monthly Acute Medication Use Days** across 12 weeks** | ||||
|
Baseline |
6.6 |
6.7 |
6.9 |
6.5 |
|
Mean change from baseline |
-3.7 |
-3.7 |
-3.9 |
-2.4 |
|
Difference from placebo |
-1.3 |
-1.3 |
-1.5 | |
|
p-value |
<0.001 |
<0.001 |
<0.001 | |
|
≥ 50%MMD Responders****across 12 weeks | ||||
|
% Responders |
56 |
59 |
61 |
29 |
|
Difference from placebo (%) |
27 |
30 |
32 | |
|
p-value |
<0.001 |
<0.001 |
<0.001 | |
|
MSQ** v2.1 RFR******Domain* at week 12 | ||||
|
Baseline |
44.9 |
44.0 |
46.8 |
46.8 |
|
Mean change from baseline |
30.4 |
30.5 |
31.3 |
20.5 |
|
Difference from placebo |
9.9 |
10.1 |
10.8 | |
|
p-value |
<0.001 |
<0.001 |
<0.001 | |
|
AIM-D****PDA Domain across 12 weeks** | ||||
|
Baseline |
15.5 |
16.9 |
15.9 |
15.2 |
|
Mean change from baseline |
-7.3 |
-8.6 |
-9.4 |
-6.1 |
|
Difference from placebo |
-1.2 |
-2.5 |
-3.3 | |
|
p-value |
NS† |
<0.001 |
<0.001 | |
|
AIM-D P**I Domain across 12 weeks* | ||||
|
Baseline |
11.7 |
13.0 |
11.6 |
11.2 |
|
Mean change from baseline |
-5.1 |
-6.0 |
-6.5 |
-4.0 |
|
Difference from placebo |
-1.1 |
-2.0 |
-2.5 | |
|
p-value |
NS† |
0.002 |
<0.001 | |
|
Figure 1 shows the mean change from baseline in MMD in Study 1. Patients treated with QULIPTA had greater mean decreases from baseline in MMD across the 12-week treatment period compared to patients who received placebo.
Figure1**: Change from Baseline in Monthly Migraine Days in Study 1**
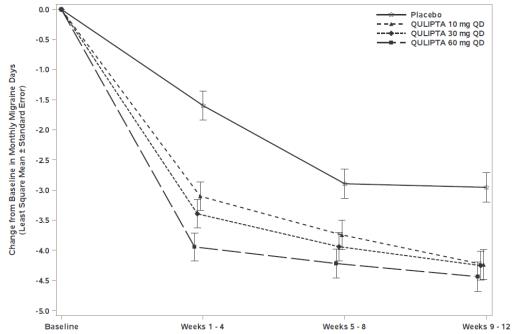
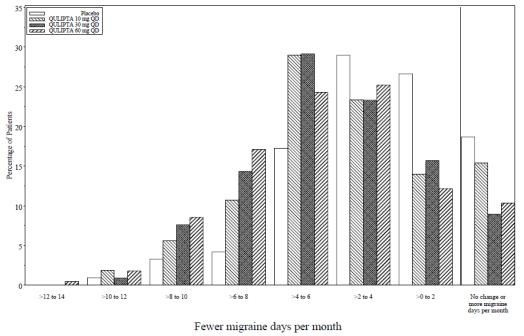
Figure 2 shows the distribution of change from baseline in mean MMD across the 12-week treatment period, in 2-day increments, by treatment group. A treatment benefit over placebo for all doses of QULIPTA is seen across a range of mean changes from baseline in MMD.
Figure2**: Distribution of Change from Baseline in Mean Monthly Migraine Days by Treatment Group in Study 1**
Study 2
The primary efficacy endpoint was the change from baseline in mean monthly migraine days across the 12-week treatment period.
Patients had a mean age of 40 years (range: 18 to 74 years), 87% were female, 76% were White, 20% were Black, and 15% were of Hispanic or Latino ethnicity. The mean migraine frequency at baseline was approximately 8 migraine days per month. A total of 541 (83%) patients completed the 12-week double-blind study period.
In Study 2, there was a significantly greater reduction in mean monthly migraine days across the 12-week treatment period in all three QULIPTA treatment groups, compared with placebo, as summarized in Table 4.
Table 4: Efficacy Endpoints in Study 2|
QULIPTA |
QULIPTA |
QULIPTA |
Placebo | |
|
Monthly Migraine Days (MMD) across 12 weeks | ||||
|
Baseline |
7.6 |
7.6 |
7.7 |
7.8 |
|
Mean change from baseline |
-4.0 |
-3.8 |
-3.6 |
-2.8 |
|
Difference from placebo |
-1.1 |
-0.9 |
-0.7 | |
|
p-value |
0.024 |
0.039 |
0.039 | |
|
Monthly Headache Days across 12 weeks | ||||
|
Baseline |
8.9 |
8.7 |
8.9 |
9.1 |
|
Mean change from baseline |
-4.3 |
-4.2 |
-3.9 |
-2.9 |
|
Difference from placebo |
-1.4 |
-1.2 |
-0.9 | |
|
p-value |
0.024 |
0.039 |
0.039 |
Figure 3 shows the mean change from baseline in MMD in Study 2. Patients treated with QULIPTA had greater mean decreases from baseline in MMD across the 12-week treatment period compared to patients who received placebo.
Figure 3: Change from Baseline in Monthly Migraine Days in Study 2
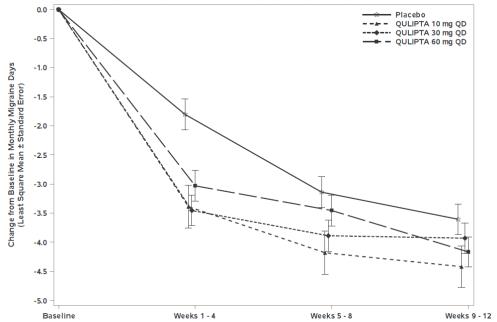
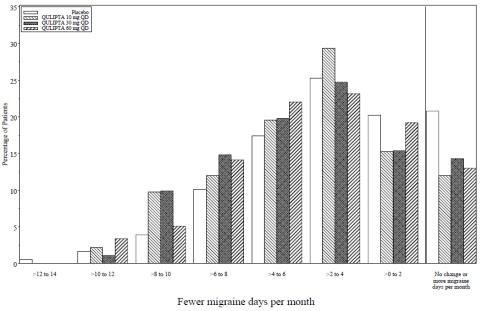
Figure 4 shows the distribution of change from baseline in mean MMD across the 12-week treatment period, in 2-day increments, by treatment group. A treatment benefit over placebo for all doses of QULIPTA is seen across a range of mean changes from baseline in MMD.
Figure 4: Distribution of Change from Baseline in Mean Monthly Migraine Days by Treatment Group in Study 2
14.2** Chronic Migraine**
Study 3
The efficacy of QULIPTA for the preventive treatment of chronic migraine in adults was demonstrated in a randomized, multicenter, double-blind, placebo- controlled study (Study 3). The study enrolled patients with at least a 1-year history of chronic migraine, according to the ICHD-3 diagnostic criteria.
Study 3 (NCT03855137) included randomization of patients to QULIPTA 60 mg once daily (N = 262) or placebo (N = 259) for 12 weeks. A subset of patients (11%) was allowed to use one concomitant migraine preventive medication. Patients were allowed to use acute headache treatments (i.e., triptans, ergotamine derivatives, NSAIDs, acetaminophen, and opioids) as needed. Patients with medication overuse headache also were enrolled. The use of a concomitant medication that acts on the CGRP pathway was not permitted for either acute or preventive treatment of migraine. The study excluded patients with myocardial infarction, stroke, or transient ischemic attacks within six months prior to screening.
The primary efficacy endpoint was the change from baseline in mean MMD across the 12-week treatment period. Secondary endpoints included the change from baseline in mean monthly headache days, the change from baseline in mean monthly acute medication use days, the proportion of patients achieving at least a 50% reduction from baseline in mean MMD (3-month average), the change from baseline in mean monthly AIM-D PDA domain scores, the change from baseline in mean monthly AIM-D PI domain scores, across the 12-week treatment period, and the change from baseline at Week 12 for MSQ v2.1 RFR domain scores.
Patients had a mean age of 42 years (range 18 to 74 years), 87% were female, 60% were White, 3% were Black, 36% were Asian, and 4% were of Hispanic or Latino ethnicity. The mean migraine frequency at baseline was approximately 19 migraine days per month and was similar across treatment groups. A total of 463 (89%) of these patients completed the 12-week double-blind study period.
Key efficacy results of Study 3 are summarized in Table 5.
Table 5: Efficacy Endpoints in Study 3|
QULIPTA****60****mg** QD** |
Placebo | |
|
Monthly Migraine Days** (MMD) across 12 weeks** | ||
|
Baseline |
19.2 |
18.9 |
|
Mean change from baseline |
-6.9 |
-5.1 |
|
Difference from placebo |
-1.8 | |
|
p-value |
<0.001 | |
|
Monthly Headache Days** across 12 weeks** | ||
|
Baseline |
21.5 |
21.4 |
|
Mean change from baseline |
-7.0 |
-5.1 |
|
Difference from placebo |
-1.9 | |
|
p-value |
<0.001 | |
|
Monthly Acute Medication Use Days** across 12 weeks** | ||
|
Baseline |
15.5 |
15.4 |
|
Mean change from baseline |
-6.2 |
-4.1 |
|
Difference from placebo |
-2.1 | |
|
p-value |
<0.001 | |
|
≥ 50%MMD Responders****across 12 weeks | ||
|
% Responders |
41 |
26 |
|
Difference from placebo (%) |
15 | |
|
p-value |
<0.001 | |
|
MSQ v2.1 RFR Domain at week 12* | ||
|
Baseline |
43.4 |
43.9 |
|
Mean change from baseline |
23.3 |
17.2 |
|
Difference from placebo |
6.2 | |
|
p-value |
<0.001 | |
|
AIM-D****PDA Domain across 12 weeks** | ||
|
Baseline |
31.2 |
29.5 |
|
Mean change from baseline |
-12.8 |
-9.4 |
|
Difference from placebo |
-3.4 | |
|
p-value |
<0.001 | |
|
AIM-D P**I Domain across 12 weeks* | ||
|
Baseline |
27.1 |
25.2 |
|
Mean change from baseline |
-10.6 |
-7.9 |
|
Difference from placebo |
-2.7 | |
|
p-value |
0.003 |
- Migraine Specific Quality of Life Questionnaire version 2.1 Role Function-Restrictive domain score
** Activity Impairment in Migraine-Diary Performance of Daily Activities domain score
*** Activity Impairment in Migraine-Diary Physical Impairment domain score
Figure 5 shows the mean change from baseline in MMD in Study 3. Patients treated with QULIPTA had greater mean decreases from baseline in MMD across the 12-week treatment period compared to patients who received placebo.
Figure** 5****: Change from Baseline in Monthly Migraine Days in Study 3**
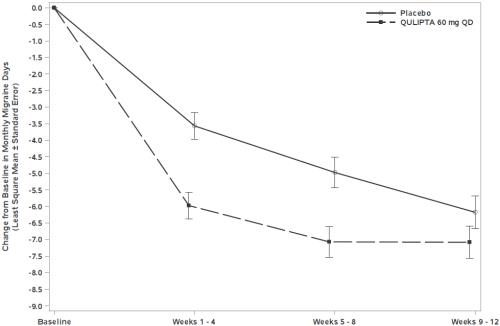
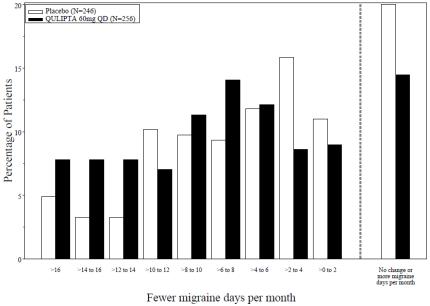
Figure 6 shows the distribution of change from baseline in mean MMD across the 12-week treatment period, in 2-day increments, by treatment group. A treatment benefit of QULIPTA over placebo is seen across a range of mean changes from baseline in MMD.
Figure** 6****: Distribution of Change from Baseline in Mean Monthly**** ****MigraineDaysby******Treatment Group in Study 3
HOW SUPPLIED SECTION
16****HOW SUPPLIED/STORAGE AND HANDLING
16.1****How Supplied
QULIPTA 10 mg is supplied as white to off-white, round biconvex tablets debossed with “A” and “10” on one side in the following packaging presentations:
- Bottle of 30, NDC: 0074-7095-30
QULIPTA 30 mg is supplied as white to off-white, oval biconvex tablets debossed with “A30” on one side in the following packaging presentations:
- Bottle of 30, NDC: 0074-7096-30
QULIPTA 60 mg is supplied as white to off-white, oval biconvex tablets debossed with “A60” on one side in the following packaging presentations:
- Bottle of 30, NDC: 0074-7094-30
16.2****Storage and Handling
Store between 20°C and 25°C (68°F and 77°F): excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17****PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions and that these reactions can occur with QULIPTA. Advise patients to discontinue QULIPTA and seek immediate medical attention if they experience any symptoms of a hypersensitivity reaction [see Warnings and Precautions (5.1)].
Drug Interactions
Inform patients that QULIPTA may interact with certain other drugs, and that dosage modifications of QULIPTA may be recommended when used with some other drugs. Advise patients to report to their healthcare provider the use of any other prescription medications, over-the-counter medications, herbal products, or grapefruit juice [see Dosage and Administration (2.2) and Drug Interactions (7.1, 7.2, 7.3)].
Manufactured for**:**
AbbVie Inc.
North Chicago, IL 60064
© 2023 AbbVie. All rights reserved.
QULIPTA and its design are trademarks of Allergan Pharmaceuticals
International Limited, an AbbVie company.
20078877 June 2023
