Clopidogrel
These highlights do not include all the information needed to use CLOPIDOGREL TABLETS safely and effectively. See full prescribing information for CLOPIDOGREL TABLETS. CLOPIDOGREL tablets, for oral use Initial U.S. Approval: 1997
65ea4dc6-acb2-4609-9a4c-4866ef0ccb11
HUMAN PRESCRIPTION DRUG LABEL
Aug 10, 2023
American Health Packaging
DUNS: 929561009
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clopidogrel Bisulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Clopidogrel Bisulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 300 mg – 6 UD

Clopidogrel
Tablet, USP
300 mg
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
- Clopidogrel tablets are indicated to reduce the rate of myocardial infarction (MI) and stroke in patients with non-ST-segment elevation ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those who are to be managed with coronary revascularization. Clopidogrel should be administered in conjunction with aspirin.
- Clopidogrel tablets are indicated to reduce the rate of myocardial infarction and stroke in patients with acute ST-elevation myocardial infarction (STEMI) who are to be managed medically. Clopidogrel tablets should be administered in conjunction with aspirin.
1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
In patients with established peripheral arterial disease or with a history of recent myocardial infarction (MI) or recent stroke clopidogrel tablets are indicated to reduce the rate of MI and stroke.
Clopidogrel tablets is a P2Y 12 platelet inhibitor indicated for:
- Acute coronary syndrome
- For patients with non-ST-segment elevation ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), clopidogrel tablets have been shown to reduce the rate of myocardial infarction (MI) and stroke. (1.1)
- For patients with ST-elevation myocardial infarction (STEMI), clopidogrel tablets have been shown to reduce the rate of MI and stroke. (1.1)
- Recent MI, recent stroke, or established peripheral arterial disease. Clopidogrel tablets have been shown to reduce the rate of MI and stroke. (1.2)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y 12 class of ADP receptors on platelets.
12.2 Pharmacodynamics
Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y 12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to clopidogrel's active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP.
Dose-dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of clopidogrel. Repeated doses of 75 mg clopidogrel per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg clopidogrel per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days.
Geriatric Patients
Elderly (≥75 years) and young healthy subjects had similar effects on platelet
aggregation.
Renally Impaired Patients
After repeated doses of 75 mg clopidogrel per day, patients with severe renal
impairment (creatinine clearance from 5 to 15 mL/min) and moderate renal
impairment (creatinine clearance from 30 to 60 mL/min) showed low (25%)
inhibition of ADP-induced platelet aggregation.
Hepatically Impaired Patients
After repeated doses of 75 mg clopidogrel per day for 10 days in patients with
severe hepatic impairment, inhibition of ADP-induced platelet aggregation was
similar to that observed in healthy subjects.
Gender
In a small study comparing men and women, less inhibition of ADP-induced
platelet aggregation was observed in women.
12.3 Pharmacokinetics
Clopidogrel is a prodrug and is metabolized to a pharmacologically active metabolite and inactive metabolites.
Absorption
After single and repeated oral doses of 75 mg per day, clopidogrel is rapidly
absorbed. Absorption is at least 50%, based on urinary excretion of
clopidogrel metabolites.
Effect of Food
Clopidogrel can be administered with or without food. In a study in healthy
male subjects when clopidogrel 75 mg per day was given with a standard
breakfast, mean inhibition of ADP-induced platelet aggregation was reduced by
less than 9%. The active metabolite AUC 0–24 was unchanged in the presence of
food, while there was a 57% decrease in active metabolite C max.
Similar results were observed when a clopidogrel 300 mg loading dose was administered with a high-fat breakfast.
Metabolism
Clopidogrel is extensively metabolized by two main metabolic pathways: one
mediated by esterases and leading to hydrolysis into an inactive carboxylic
acid derivative (85% of circulating metabolites) and one mediated by multiple
cytochrome P450 enzymes. Cytochromes first oxidize clopidogrel to a 2-oxo-
clopidogrel intermediate metabolite. Subsequent metabolism of the 2-oxo-
clopidogrel intermediate metabolite results in formation of the active
metabolite, a thiol derivative of clopidogrel. The active metabolite is formed
mostly by CYP2C19 with contributions from several other CYP enzymes, including
CYP1A2, CYP2B6 and CYP3A. The active thiol metabolite binds rapidly and
irreversibly to platelet receptors, thus inhibiting platelet aggregation for
the lifespan of the platelet.
The C max of the active metabolite is twice as high following a single 300 mg clopidogrel loading dose as it is after four days of 75 mg maintenance dose. C max occurs approximately 30 to 60 minutes after dosing. In the 75 to 300 mg dose range, the pharmacokinetics of the active metabolite deviates from dose proportionality: 4-fold the dose results in 2.0-fold and 2.7-fold the C max and AUC, respectively.
Elimination
Following an oral dose of 14C-labeled clopidogrel in humans, approximately 50%
of total radioactivity was excreted in urine and approximately 46% in feces
over the 5 days post-dosing. After a single, oral dose of 75 mg, clopidogrel
has a half-life of approximately 6 hours. The half-life of the active
metabolite is about 30 minutes.
Drug Interactions
Effect of other drugs on Clopidogrel
Clopidogrel is metabolized to its active metabolite in part by CYP2C19.
CYP2C19 inducers
Concomitant use of strong inducers of CYP2C19 results in increased plasma
concentration of the active metabolite of clopidogrel and an increase in
platelet inhibition.
Rifampin: Coadministration of rifampin 300 mg twice daily for 7 days with 600 mg loading dose of clopidogrel in healthy adults increased the mean AUC and C max of clopidogrel’s thiol metabolites by 3.8-fold. Mean inhibition of platelet aggregation at 4 hours post-dose was 34% higher in the presence of rifampin compared to clopidogrel administered alone.
CYP2C19 inhibitors
Concomitant use of certain inhibitors of this enzyme results in reduced plasma
concentrations of the active metabolite of clopidogrel and a reduction in
platelet inhibition.
Proton pump inhibitors (PPI)
The effect of proton pump inhibitors (PPI) on the systemic exposure to the
clopidogrel active metabolite following multiple doses of clopidogrel 75 mg
evaluated in dedicated drug interaction studies is presented in Figure 1.
Figure 1: Exposure to Clopidogrel Active Metabolite Following Multiple Doses of Clopidogrel 75 mg Alone or with Proton Pump Inhibitors (PPIs)
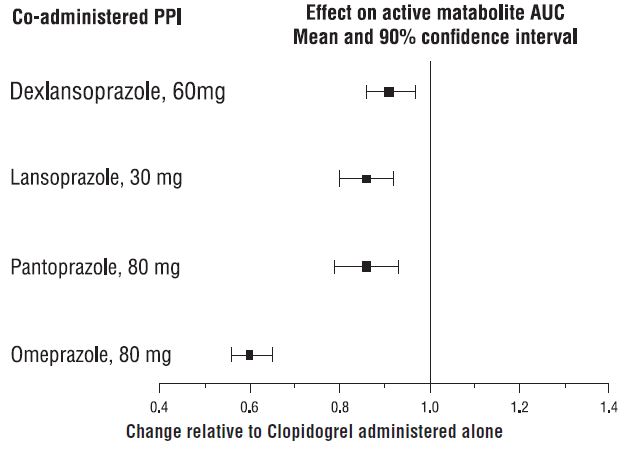
Pharmacodynamic and pharmacokinetic parameters measured in these studies showed that the interaction was highest with omeprazole and least with dexlansoprazole.
Opioids
Coadministration of 5 mg intravenous morphine with 600 mg loading dose of
clopidogrel in healthy adults decreased the AUC and C max of clopidogrel’s
thiol metabolites by 34%. Mean platelet aggregation was higher up to 2 to 4
hours with morphine coadministration.
Effect of clopidogrel on other drugs
In vitro studies have shown that the glucuronide metabolite of clopidogrel is
a strong inhibitor of CYP2C8. Concomitant administration of repaglinide with
clopidogrel increased the systemic exposure to repaglinide (AUC 0-∞) by
5.1-fold following the loading dose (300 mg) and by 3.9-fold on day 3 of the
maintenance dose (75 mg) of clopidogrel [see Drug Interactions (7.8)].
12.5 Pharmacogenomics
CYP2C19 is involved in the formation of both the active metabolite and the 2-oxo-clopidogrel intermediate metabolite. Clopidogrel active metabolite pharmacokinetics and antiplatelet effects, as measured by ex vivo platelet aggregation assays, differ according to CYP2C19 genotype. Patients who are homozygous for nonfunctional alleles of the CYP2C19 gene are termed "CYP2C19 poor metabolizers". Approximately 2% of White and 4% of Black patients are poor metabolizers; the prevalence of poor metabolism is higher in Asian patients (e.g., 14% of Chinese). Tests are available to identify patients who are CYP2C19 poor metabolizers.
A crossover study in 40 healthy subjects, 10 each in the four CYP2C19 metabolizer groups, evaluated pharmacokinetic and antiplatelet responses using 300 mg followed by 75 mg per day and 600 mg followed by 150 mg per day, each for a total of 5 days. Decreased active metabolite exposure and diminished inhibition of platelet aggregation were observed in the poor metabolizers as compared to the other groups.
Table 3: Active Metabolite Pharmacokinetics and Antiplatelet Responses by CYP2C19 Metabolizer Status
| |||||
|
Dose |
Poor (n=10) |
Intermediate* (n=10) |
Normal (n=10) |
Ultrarapid† (n=10) | |
|
C max (ng/mL) |
300 mg (24 h) 600 mg (24 h) 75 mg (Day 5) 150 mg (Day 5) |
11 (4) 17 (6) 4 (1) 7 (2) |
23 (11) 39 (23) 12 (5) 18 (7) |
32 (21) 44 (27) 13 (7) 19 (5) |
24 (10) 36 (13) 12 (6) 16 (9) |
|
IPA (%) ‡ |
300 mg (24 h) 600 mg (24 h) 75 mg (Day 5) 150 mg (Day 5) |
24 (26) 32 (25) 37 (23) 61 (14) |
37 (21) 56 (22) 60 (18) 74 (14) |
39 (28) 49 (23) 58 (19) 73 (9) |
40 (21) 51 (28) 56 (13) 68 (18) |
|
VASP-PRI (%) § |
300 mg (24 h) 600 mg (24 h) 75 mg (Day 5) 150 mg (Day 5) |
91 (12) 85 (14) 83 (13) 61 (18) |
78 (12) 56 (26) 50 (16) 29 (11) |
68 (16) 48 (20) 39 (14) 24 (10) |
73 (12) 51 (20) 40 (9) 20 (10) |
Values are mean (SD)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 mg/kg per day, which afforded plasma exposures >25 times that in humans at the recommended daily dose of 75 mg.
Clopidogrel was not genotoxic in four in vitro tests (Ames test, DNA-repair test in rat hepatocytes, gene mutation assay in Chinese hamster fibroblasts, and metaphase chromosome analysis of human lymphocytes) and in one in vivo test (micronucleus test by oral route in mice).
Clopidogrel was found to have no effect on fertility of male and female rats treated prior to pairing and throughout gestation at oral doses up to 400 mg/kg per day (52 times the recommended human dose on a mg/m 2 basis).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read FDA approved patient labeling (Medication Guide).
Discontinuation
Advise patients not to discontinue clopidogrel without first discussing it
with the healthcare provider who prescribed it [see Warnings and Precautions (5.3)].
Bleeding
Advise patients that they:
- will bruise and bleed more easily
- will take longer than usual to stop bleeding
- must report any unanticipated, prolonged, or excessive bleeding, or blood in their stool or urine [see Warnings and Precautions (5.2)].
Thrombotic Thrombocytopenic Purpura
Instruct patients to get prompt medical attention if they experience symptoms
of TTP that cannot otherwise be explained [see Warnings and Precautions (5.4)].
Invasive Procedures
Advise patients to inform physicians and dentists that they are taking
clopidogrel before any surgery or dental procedure [see Warnings and Precautions (5.2, 5.3)].
Proton Pump Inhibitors
Advise patients not to take omeprazole or esomeprazole while taking
clopidogrel. Dexlansoprazole, lansoprazole and pantoprazole had less
pronounced effects on the antiplatelet activity of clopidogrel than did
omeprazole or esomeprazole [see Drug Interactions (7.2)].
Dispense with Medication Guide.
To order more Medication Guides call American Health Packaging at
1‐800‐707‐4621.
