Timolol Maleate
{'content': {'@styleCode': 'bold'}}
57c62928-63d8-4505-9b9e-c085a3a12c95
HUMAN PRESCRIPTION DRUG LABEL
Jul 6, 2020
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
timolol maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
timolol maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
timolol maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
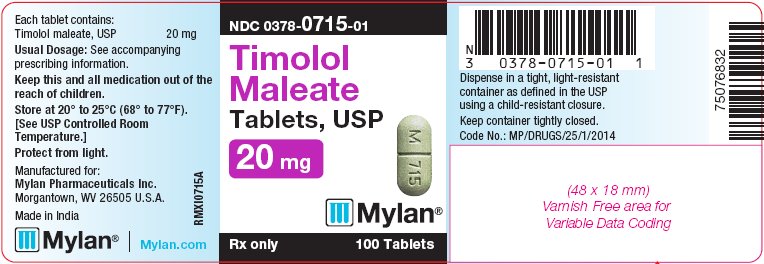
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 20 mg
NDC 0378-0715-01
Timolol
Maleate
Tablets, USP
20 mg
Rx only 100 Tablets
Each tablet contains:
Timolol maleate, USP 20 mg
Usual Dosage: See accompanying
prescribing information.
Keep this and all medication out of the
reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light.
Manufactured for:
Mylan Pharmaceuticals Inc.
****Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXI0715A
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MP/DRUGS/25/1/2014
DESCRIPTION SECTION
DESCRIPTION
Timolol maleate is a nonselective beta-adrenergic receptor blocking agent. The chemical name for timolol maleate is (S)-1-[(1,1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-2-propanol (Z)-2-butenedioate (1:1) salt. It possesses an asymmetric carbon atom in its structure and is provided as the levo isomer. Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is:

Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol.
Timolol maleate is supplied as tablets containing 5 mg, 10 mg and 20 mg timolol maleate for oral administration. Inactive ingredients are: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch (corn), sodium lauryl sulfate, FD&C Blue No. 2 aluminum lake, and D&C Yellow No. 10 aluminum lake.
