Diflorasone Diacetate
Diflorasone Diacetate Ointment USP, 0.05% Rx Only For Topical Use Only – Not For Ophthalmic Use
23eb27a4-157f-4eb5-a390-37586710808f
HUMAN PRESCRIPTION DRUG LABEL
May 2, 2023
Rising Pharma Holdings, Inc.
DUNS: 116880195
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
diflorasone diacetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Each gram of diflorasone diacetate ointment contains 0.5 mg diflorasone diacetate in an ointment base. Chemically, diflorasone diacetate is 6α,9-difluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3, 20-dione 17,21-diacetate. The structural formula is represented below:
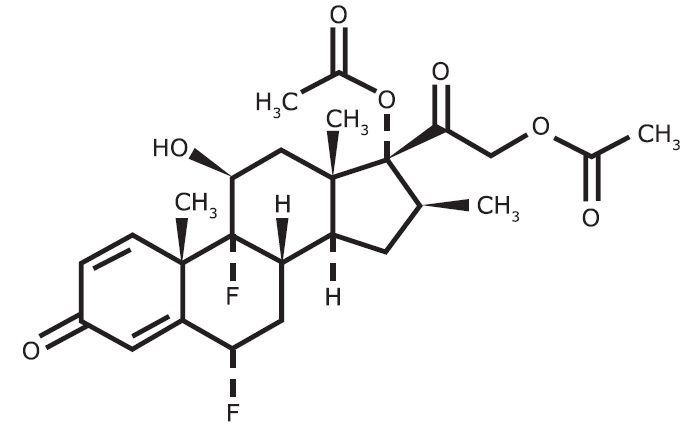
C26H32F2O7 MW = 494.54
Each gram of diflorasone diacetate ointment contains 0.5 mg diflorasone diacetate in an ointment base of propylene glycol, glyceryl monostearate and white petrolatum.
HOW SUPPLIED SECTION
HOW SUPPLIED
Diflorasone Diacetate Ointment USP, 0.05% is available in 15 gram, 30 gram, 45 gram and 60 gram tubes.
|
15 gram tube |
NDC 64980-124-15 |
|
30 gram tube |
NDC 64980-124-03 |
|
45 gram tube |
NDC 64980-124-45 |
|
60 gram tube |
NDC 64980-124-60 |
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Keep this and all medications out of the reach of children.
Mfd. By:
Lyne Laboratories, Inc.
Brockton, MA 02301
Mfd. For:
Rising Pharmaceuticals, Inc.
East Brunswick, NJ 08816
R2-03/23
PIR12460-01
