Levothyroxine sodium
These highlights do not include all the information needed to use LEVOTHYROXINE SODIUM TABLETS safely and effectively. See full prescribing information for LEVOTHYROXINE SODIUM TABLETS. LEVOTHYROXINE SODIUM tablets, for oral use Initial U.S. Approval: 2002
da570872-94a1-4852-e053-2995a90aacb6
HUMAN PRESCRIPTION DRUG LABEL
Jan 1, 2023
Northwind Pharmaceuticals
DUNS: 036986393
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Levothyroxine Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with
Underlying Cardiovascular Disease
Over-treatment with levothyroxine may cause an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias, particularly in patients with cardiovascular disease and in elderly patients. Initiate levothyroxine sodium therapy in this population at lower doses than those recommended in younger individuals or in patients without cardiac disease [see Dosage and Administration (2.3), Use in Specific Populations (8.5)] .
Monitor for cardiac arrhythmias during surgical procedures in patients with coronary artery disease receiving suppressive levothyroxine sodium therapy. Monitor patients receiving concomitant levothyroxine sodium and sympathomimetic agents for signs and symptoms of coronary insufficiency.
If cardiac symptoms develop or worsen, reduce the levothyroxine sodium tablets dose or withhold for one week and restart at a lower dose.
5.2 Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism, and may result in unpredictable absorption of levothyroxine sodium from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with levothyroxine sodium [see Contraindications (4)] .
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
Levothyroxine sodium has a narrow therapeutic index. Over- or under treatment with levothyroxine sodium may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, emotional state, gastrointestinal function, and glucose and lipid metabolism. Titrate the dose of levothyroxine sodium carefully and monitor response to titration to avoid these effects [see Dosage and Administration (2.4)] . Monitor for the presence of drug or food interactions when using levothyroxine sodium and adjust the dose as necessary [see Drug Interactions (7.9) and Clinical Pharmacology (12.3)] .
5.5 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing levothyroxine sodium [see Drug Interactions (7.2)] .
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-
Replacement
Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in post-menopausal women. The increased bone resorption may be associated with increased serum levels and urinary excretion of calcium and phosphorous, elevations in bone alkaline phosphatase, and suppressed serum parathyroid hormone levels. Administer the minimum dose of levothyroxine sodium that achieves the desired clinical and biochemical response to mitigate this risk.
5.7 Risk of Allergic Reactions Due to Tartrazine
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic- type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
- Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate levothyroxine sodium at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation. (2.3, 5.1, 8.5)
- Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.2)
- Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of levothyroxine sodium treatment. (5.3)
- Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism. (5.4)
- Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy. (5.5)
- Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose. (5.6)
OVERDOSAGE SECTION
10 OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)] . In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures occurred in a 3-year-old child ingesting 3.6 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Reduce the levothyroxine sodium dose or discontinue temporarily if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient’s medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
DESCRIPTION SECTION
11 DESCRIPTION
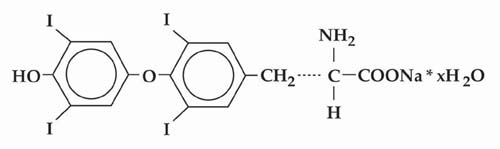
Levothyroxine sodium tablets, USP contain synthetic crystalline L-3,3',5,5'-tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C 15H 10I 4N NaO 4• H 2O, molecular weight of 798.86 (anhydrous), and structural formula as shown:
Levothyroxine sodium tablets, USP for oral administration are supplied in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg, and 300 mcg. Each levothyroxine sodium tablet contains the inactive ingredients microcrystalline sodium, light magnesium oxide, sodium starch glycolate and sodium stearyl fumarate.
Levothyroxine sodium tablets, USP contain no ingredients made from a gluten- containing grain (wheat, barley, or rye). Table 6 provides a listing of the color additives by tablet strength:
|
Table 6. Levothyroxine Sodium Tablets Color Additives | |
|
Strength |
Color additive(s) |
|
25 |
FD&C Yellow No. 6 Aluminum Lake |
|
50 |
None |
|
75 |
FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake |
|
88 |
FD&C Blue No. 2 Aluminum Lake, FD&C Yellow No. 5 Aluminum Lake |
|
100 |
FD&C Yellow No. 5 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake |
|
112 |
FD&C Red No. 40 Aluminum Lake, Carmine |
|
125 |
FD&C Yellow No. 6 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake |
|
137 |
FD&C Blue No. 1 Aluminum Lake |
|
150 |
FD&C Blue No. 2 Aluminum Lake |
|
175 |
FD&C Blue No. 1 Aluminum Lake, Carmine |
|
200 |
FD&C Red No. 40 Aluminum Lake |
|
300 |
D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake |
FDA approved dissolution test method differs from the USP dissolution test methods.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Oral levothyroxine sodium is a synthetic T4 hormone that exerts the same physiologic effect as endogenous T4, thereby maintaining normal T4 levels when a deficiency is present.
12.3 Pharmacokinetics
Absorption
Absorption of orally administered T4 from the gastrointestinal tract ranges from 40% to 80%. The majority of the levothyroxine sodium dose is absorbed from the jejunum and upper ileum. The relative bioavailability of levothyroxine sodium tablets, compared to an equal nominal dose of oral levothyroxine sodium solution, is approximately 93%. T4 absorption is increased by fasting, and decreased in malabsorption syndromes and by certain foods such as soybeans. Dietary fiber decreases bioavailability of T4. Absorption may also decrease with age. In addition, many drugs and foods affect T4 absorption [see Drug Interactions (7)] .
Distribution
Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA), and albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T4 partially explains the higher serum levels, slower metabolic clearance, and longer half-life of T4 compared to T3. Protein-bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Drug Interactions (7)] . Thyroid hormones do not readily cross the placental barrier [see Use in Specific Populations (8.1)] .
Elimination
Metabolism
T4 is slowly eliminated (see Table 7). The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately 80% of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (rT3). T3 and rT3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Excretion
Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged and is eliminated in the feces. Approximately 20% of T4 is eliminated in the stool. Urinary excretion of T4 decreases with age.
|
Table 7. Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients | ||||
|
Hormone |
Ratio in Thyroglobulin |
Biologic Potency |
t1/2 (days) |
**Protein Binding (%)**a |
|
Levothyroxine (T4) |
10 to 20 |
1 |
6 to 7 b |
99.96 |
|
Liothyronine (T3) |
1 |
4 |
≤ 2 |
99.5 |
|
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of levothyroxine.
