Valacyclovir Hydrochloride
These highlights do not include all the information needed to use VALACYCLOVIR TABLETS safely and effectively. See full prescribing information for VALACYCLOVIR TABLETS. VALACYCLOVIR tablets, for oral use Initial U.S. Approval: 1995
ae8d15a4-1ed5-4e3c-9672-0f4735322a1c
HUMAN PRESCRIPTION DRUG LABEL
Jun 18, 2025
PD-Rx Pharmaceuticals, Inc.
DUNS: 156893695
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Valacyclovir Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg
Rx only
Valacyclovir Tablets, USP
500 mg

DESCRIPTION SECTION
11 DESCRIPTION
Valacyclovir hydrochloride is the hydrochloride salt of the L-valyl ester of the antiviral drug acyclovir.
Valacyclovir tablets, USP are for oral administration. Each tablet contains 556 mg or 1.112 grams of valacyclovir hydrochloride USP (hydrous) equivalent to 500 mg or 1 gram of valacyclovir, respectively, and the inactive ingredients crospovidone, FD&C blue #2/indigo carmine aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide.
The chemical name of valacyclovir hydrochloride is L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H-purin-9-yl)methoxy]ethyl ester, monohydrochloride. It has the following structural formula:
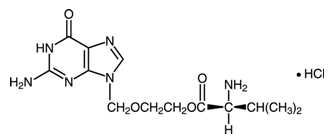
Valacyclovir hydrochloride USP (hydrous) is a white or almost white powder with the molecular formula C 13H 20N 6O 4•HCl and a molecular weight of 360.80. The maximum solubility in water at 25°C is 174 mg/mL. The pk as for valacyclovir hydrochloride are 1.90, 7.47, and 9.43.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Valacyclovir Tablets USP, 500 mgare blue, film-coated, capsule shaped tablets with “F 82” on one side and plain on the otherside containing 556 mg of valacyclovir hydrochloride equivalent to 500 mg of valacyclovir.
Bottles of 6 NDC 72789-263-06
Bottles of 10 NDC 72789-263-10
Bottles of 14 NDC 72789-263-14
Bottles of 28 NDC 72789-263-28
Bottles of 90 NDC 72789-263-90
Store at20 oto 25 oC (68 oto 77 oF). [See USP Controlled Room Temperature.]
Dispense in a well-closed container as defined in the USP.
