Norgestimate and Ethinyl Estradiol
These highlights do not include all the information needed to use NORGESTIMATE AND ETHINYL ESTRADIOL TABLETS safely and effectively. See full prescribing information for NORGESTIMATE AND ETHINYL ESTRADIOL TABLETS. NORGESTIMATE and ETHINYL ESTRADIOL tablets, 0.25 mg/0.035 mg, for oral useNORGESTIMATE and ETHINYL ESTRADIOL tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg, for oral use Initial U.S. Approval: 1989
6ae97238-28cf-4bc9-a4ba-1e2bf4e3c600
HUMAN PRESCRIPTION DRUG LABEL
Mar 28, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Norgestimate and Ethinyl Estradiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Oral Contraceptive
Norgestimate and Ethinyl Estradiol Tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg and Norgestimate and Ethinyl Estradiol Tablets, 0.25 mg/0.035 mg are indicated for use by females of reproductive potential to prevent pregnancy [see Clinical Studies (14)].
1.2 Acne
Norgestimate and Ethinyl Estradiol Tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg are indicated for the treatment of moderate acne vulgaris in females at least 15 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Norgestimate and Ethinyl Estradiol Tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control [see Clinical Studies (14)].
Norgestimate and ethinyl estradiol tablets are estrogen/progestin COCs, indicated for use by women to prevent pregnancy. (1.1)
Norgestimate and ethinyl estradiol tablets 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg are also indicated for the treatment of moderate acne vulgaris in females at least 15 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche.
Norgestimate and ethinyl estradiol tablets 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control. (1.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of COCs are discussed elsewhere in labeling:
•
Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions (5.1)]
•
Vascular events [see Warnings and Precautions (5.1)]
•
Liver disease [see Warnings and Precautions (5.2)]
Adverse reactions commonly reported by COC users are:
•
Irregular uterine bleeding
•
Nausea
•
Breast tenderness
•
Headache
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Norgestimate and Ethinyl Estradiol Tablets, 0.25 mg/0.035 mg
The safety of norgestimate and ethinyl estradiol tablets, 0.25 mg/0.035 mg, was evaluated in 1,647 healthy women of child-bearing potential who participated in 3 clinical trials and received at least 1 dose of norgestimate and ethinyl estradiol tablets, 0.25 mg/0.035 mg, for contraception. Two trials were randomized active-controlled trials and 1 was an uncontrolled open-label trial. In all 3 trials, subjects were followed for up to 24 cycles.
Common Adverse Reactions (≥ 2% of subjects): The most common adverse reactions reported by at least 2% of the 1,647 women were the following in order of decreasing incidence: headache/migraine (32.9%), abdominal/gastrointestinal pain (7.8%), vaginal infection (8.4%), genital discharge (6.8%), breast issues (including breast pain, discharge, and enlargement) (6.3%), mood disorders (including depression and mood altered) (5.0%), flatulence (3.2%), nervousness (2.9%), and rash (2.6%).
Adverse Reactions Leading to Study Discontinuation: Over the three trials, between 11 to 21% of subjects discontinued the trial due to an adverse reaction. The most common adverse reactions (≥1%) leading to discontinuation were: metrorrhagia (6.9%), nausea/vomiting (5.0%), headache (4.1%), mood disorders (including depression and mood altered) (2.4%), premenstrual syndrome (1.7%), hypertension (1.4%), breast pain (1.4%), nervousness (1.3%), amenorrhea (1.1%), dysmenorrhea (1.1%), weight increased (1.1%), and flatulence (1.1%).
Serious Adverse Reactions: breast cancer (1 subject), mood disorders including depression, irritability, and mood swings (1 subject), myocardial infarction (1 subject), and venous thromboembolic events including pulmonary embolism (1 subject) and deep vein thrombosis (DVT) (1 subject).
Norgestimate and Ethinyl Estradiol Tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg
The safety of norgestimate and ethinyl estradiol tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg, was evaluated in 4,826 healthy women of child-bearing potential who participated in 6 clinical trials and received at least 1 dose of norgestimate and ethinyl estradiol tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg, for contraception. Two trials were randomized active-controlled trials and 4 were uncontrolled open-label trials. In 3 trials, subjects were followed for up to 24 cycles; in 2 trials, subjects were followed for up to 12 cycles; and in 1 trial, subjects were followed for up to 6 cycles.
Common Adverse Reactions (≥ 2% of subjects): The most common adverse reactions reported by at least 2% of the 4,826 women were the following in order of decreasing incidence: headache/migraine (33.6%), breast issues (including breast pain, enlargement, and discharge) (8.0%), vaginal infection (7.1%), abdominal/gastrointestinal pain (5.6%), mood disorders (including mood alteration and depression) (3.8%), genital discharge (3.2%), and changes in weight (including weight fluctuation, increased or decreased) (2.5%).
Adverse Reactions Leading to Study Discontinuation: Over the trials, between 9 to 27% of subjects discontinued the trial due to an adverse reaction. The most common adverse reactions (≥1%) leading to discontinuation were: metrorrhagia (4.3%), nausea/vomiting (2.8%), headache/migraine (2.4%), mood disorders (including depression and mood altered) (1.1%), and weight increased (1.1%).
Serious Adverse Reactions: breast cancer (1 subject), carcinoma of the cervix in situ (1 subject), hypertension (1 subject), and migraine (2 subjects).
6.2 Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 to 1.12 (Figure 1).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 to 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8 to 10 years of COC use.
Figure 1
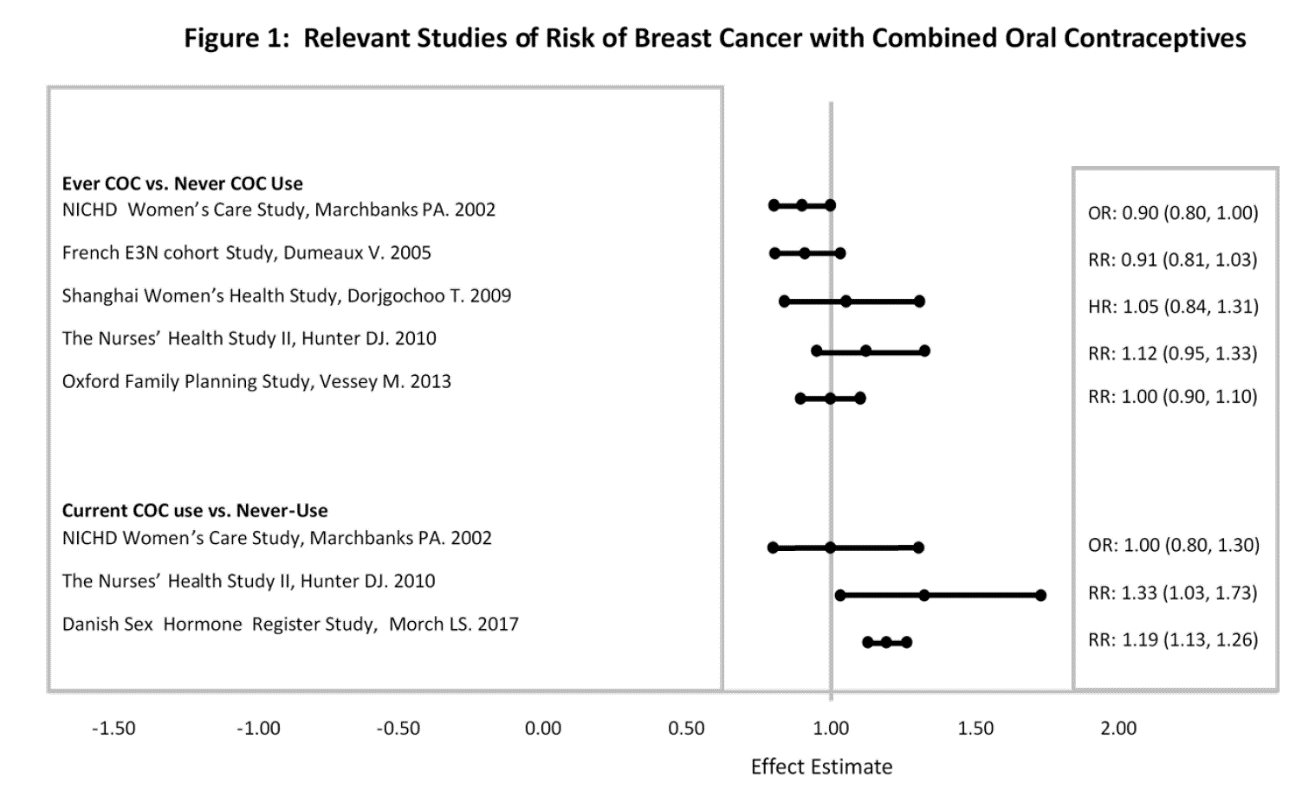
RR = relative risk;OR = odds ratio;HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following additional adverse drug reactions have been reported from worldwide postmarketing experience with norgestimate/ethinyl estradiol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and Infestations: Urinary tract infection;
Neoplasms Benign, Malignant and Unspecified (Incl. Cysts and Polyps): Breast cancer, benign breast neoplasm, hepatic adenoma, focal nodular hyperplasia, breast cyst;
Immune System Disorders: Hypersensitivity;
Metabolism and Nutrition Disorders: Dyslipidemia;
Psychiatric Disorders: Anxiety, insomnia;
Nervous System Disorders: Syncope, convulsion, paresthesia, dizziness;
Eye Disorders: Visual impairment, dry eye, contact lens intolerance;
Ear and Labyrinth Disorders: Vertigo;
Cardiac Disorders: Tachycardia, palpitations;
Vascular Events: Deep vein thrombosis, pulmonary embolism, retinal vascular thrombosis, hot flush, venous thrombosis (including Budd Chiari Syndrome and hepatic vein thrombosis);
Arterial Events: Arterial thromboembolism, myocardial infarction, cerebrovascular accident;
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea;
Gastrointestinal Disorders: Pancreatitis, abdominal distension, diarrhea, constipation;
Hepatobiliary Disorders: Hepatitis;
Skin and Subcutaneous Tissue Disorders: Angioedema, erythema nodosum, hirsutism, night sweats, hyperhidrosis, photosensitivity reaction, urticaria, pruritus, acne;
Musculoskeletal, Connective Tissue, and Bone Disorders: Muscle spasms, pain in extremity, myalgia, back pain;
Reproductive System and Breast Disorders: Ovarian cyst, suppressed lactation, vulvovaginal dryness;
General Disorders and Administration Site Conditions: Chest pain, asthenic conditions.
The most common adverse reactions reported during clinical trials (≥2%) were:
Norgestimate and ethinyl estradiol tablets, 0.25 mg/0.035 mg: headache/migraine, abdominal/gastrointestinal pain, vaginal infection, genital discharge, breast issues (including breast pain, discharge, and enlargement), mood disorders (including depression and mood altered), flatulence, nervousness, rash. (6.1)
Norgestimate and ethinyl estradiol tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg: headache/migraine, breast issues (including breast pain, enlargement, and discharge), vaginal infection, abdominal/gastrointestinal pain, mood disorders (including mood alteration and depression), genital discharge, changes in weight (including weight increased or decreased). (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There is little or no increased risk of birth defects in women who inadvertently use COCs during early pregnancy. Epidemiologic studies and meta- analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb reduction defects) following exposure to low dose COCs prior to conception or during early pregnancy.
Do not administer COCs to induce withdrawal bleeding as a test for pregnancy. Do not use COCs during pregnancy to treat threatened or habitual abortion.
8.3 Nursing Mothers
Advise the nursing mother to use other forms of contraception, when possible, until she has weaned her child. COCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women. Small amounts of oral contraceptive steroids and/or metabolites are present in breast milk.
8.4 Pediatric Use
Safety and efficacy of norgestimate and ethinyl estradiol tablets have been established in women of reproductive age. Efficacy is expected to be the same for post-pubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
There was no significant difference between norgestimate and ethinyl estradiol tablets, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg, and placebo in mean change in total lumbar spine (L1-L4) and total hip bone mineral density between baseline and Cycle 13 in 123 adolescent females with anorexia nervosa in a double-blind, placebo-controlled, multicenter, one-year treatment duration clinical trial for the Intent To Treat (ITT) population.
8.5 Geriatric Use
Norgestimate and ethinyl estradiol tablets have not been studied in postmenopausal women and are not indicated in this population.
8.6 Hepatic Impairment
The pharmacokinetics of norgestimate and ethinyl estradiol tablets have not been studied in subjects with hepatic impairment. However, steroid hormones may be poorly metabolized in patients with hepatic impairment. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded. [See Contraindications (4) and Warnings and Precautions (5.2).]
8.7 Renal Impairment
The pharmacokinetics of norgestimate and ethinyl estradiol tablets have not been studied in women with renal impairment.
Nursing mothers: Not recommended; can decrease milk production. (8.3)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Norgestimate and Ethinyl Estradiol Tablets, USP 0.25 mg/0.035 mg are available in blister packs containing 28 tablets, as follows:
•
21 blue, round, flat faced beveled edged, uncoated tablets, debossed with ‘A7’ on one side and plain on the other. Each blue tablet contains 0.25 mg of norgestimate, USP and 0.035 mg of ethinyl estradiol, USP.
•
7 light green, round, flat faced beveled edged, uncoated tablets debossed with ‘A2’on one side and plain on the other. Each light green tablet contains inert ingredients.
NDC 68462-309-29 1 carton containing 3 blister card of 28 tablets
Norgestimate and Ethinyl Estradiol Tablets, USP 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg are available in blister packs containing 28 tablets, as follows:
•
7 white to off-white, round, flat faced beveled edge, uncoated tablets, debossed with ‘A9’ on one side and plain on the other. Each white to off-white tablet contains 0.18 mg of norgestimate, USP and 0.035 mg of ethinyl estradiol, USP.
•
7 light blue, round, flat faced beveled edge, uncoated tablets, debossed with ‘A8’ on one side and plain on the other. Each light blue tablet contains 0.215 mg norgestimate, USP and 0.035 mg of ethinyl estradiol, USP.
•
7 blue, round, flat faced beveled edge, uncoated tablets, debossed with ‘A7’ on one side, and plain on the other. Each blue tablet contains 0.25 mg of norgestimate, USP and 0.035 mg of ethinyl estradiol, USP.
•
7 light green, round, flat faced beveled edge, uncoated tablets, debossed with ‘A2’ on one side and plain on the other. Each light green tablet contains inert ingredients.
NDC 68462-565-29 1 carton containing 3 blister card of 28 tablets
16.2 Storage Conditions
•
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
•
Protect from light.
•
Keep out of the reach of children.
