Exforge HCT
These highlights do not include all the information needed to use EXFORGE HCT safely and effectively. See full prescribing information for EXFORGE HCT. EXFORGE HCT (amlodipine, valsartan, and hydrochlorothiazide) tablets, for oral use Initial U.S. Approval: 2009
18d7820d-471f-4ee2-9ec6-25d8d27c77de
HUMAN PRESCRIPTION DRUG LABEL
Jul 13, 2023
Novartis Pharmaceuticals Corporation
DUNS: 002147023
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
amlodipine valsartan and hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
amlodipine valsartan and hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
amlodipine valsartan and hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
amlodipine valsartan and hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
amlodipine valsartan and hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Valsartan
Exforge HCT can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Exforge HCT as soon as possible [see Use in Specific Populations (8.1)].
Hydrochlorothiazide
Thiazides cross the placenta, and use of thiazides during pregnancy is associated with fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
5.2 Hypotension in Volume- or Salt-Depleted Patients
Excessive hypotension, including orthostatic hypotension, was seen in 1.7% of patients treated with the maximum dose of Exforge HCT (10/320/25 mg) compared to 1.8% of valsartan/HCTZ (320/25 mg) patients, 0.4% of amlodipine/valsartan (10/320 mg) patients, and 0.2% of HCTZ/amlodipine (25/10 mg) patients in a controlled trial in patients with moderate to severe uncomplicated hypertension. In patients with an activated renin-angiotensin system, such as volume- or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur in patients receiving angiotensin receptor blockers. Correct this condition prior to administration of Exforge HCT.
Exforge HCT has not been studied in patients with heart failure, recent myocardial infarction, or in patients undergoing surgery or dialysis. Patients with heart failure or post-myocardial infarction who were given valsartan commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. In controlled trials in heart failure patients, the incidence of hypotension in valsartan-treated patients was 5.5% compared to 1.8% in placebo-treated patients. In the Valsartan in Acute Myocardial Infarction Trial (VALIANT), hypotension in post-myocardial infarction patients led to permanent discontinuation of therapy in 1.4% of valsartan-treated patients and 0.8% of captopril-treated patients.
Since the vasodilation induced by amlodipine is gradual in onset, acute hypotension has rarely been reported after oral administration. Do not initiate treatment with Exforge HCT in patients with aortic or mitral stenosis or obstructive hypertrophic cardiomyopathy.
If excessive hypotension occurs with Exforge HCT, place the patient in a supine position and, if necessary, give intravenous normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.3 Increased Angina and/or Myocardial Infarction
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
5.4 Impaired Renal Function
Changes in renal function, including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin- angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on Exforge HCT. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Exforge HCT [see Drug Interactions (7)].
5.5 Potassium Abnormalities
In the controlled trial of Exforge HCT in moderate to severe hypertensive patients, the incidence of hypokalemia (serum potassium < 3.5 mEq/L) at any time post-baseline with the maximum dose of Exforge HCT (10/320/25 mg) was 10% compared to 25% with HCTZ/amlodipine (25/10 mg), 7% with valsartan/HCTZ (320/25 mg), and 3% with amlodipine/valsartan (10/320 mg). One patient (0.2%) discontinued therapy due to an adverse event of hypokalemia in each of the Exforge HCT and HCTZ/amlodipine groups. The incidence of hyperkalemia (serum potassium > 5.7 mEq/L) was 0.4% with Exforge HCT compared to 0.2% to 0.7% with the dual therapies.
Some patients with heart failure have developed increases in potassium on valsartan. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of the diuretic and/or valsartan may be required.
Hydrochlorothiazide can cause hypokalemia and hyponatremia. Hypomagnesemia can result in hypokalemia which appears difficult to treat despite potassium repletion. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Monitor serum electrolytes periodically.
If hypokalemia is accompanied by clinical signs (e.g., muscular weakness, paresis, or ECG alterations), Exforge HCT should be discontinued. Correction of hypokalemia and any coexisting hypomagnesemia is recommended prior to the initiation of thiazides.
5.6 Hypersensitivity Reaction
Hypersensitivity reactions to hydrochlorothiazide may occur in patients with or without a history of allergy or bronchial asthma, but are more likely in patients with such a history.
5.7 Systemic Lupus Erythematosus
Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
5.8 Lithium Interaction
Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of valsartan or thiazide diuretics. Monitor lithium levels in patients receiving Exforge HCT and lithium [see Drug Interactions (7)].
5.9 Metabolic Imbalances
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hydrochlorothiazide may raise the serum uric acid level due to reduced clearance of uric acid and may cause or exacerbate hyperuricemia and precipitate gout in susceptible patients.
Hydrochlorothiazide decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels in patients with hypercalcemia receiving Exforge HCT.
5.10 Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
-
Hypotension: Correct volume depletion prior to initiation (5.2)
-
Increased angina and/or myocardial infarction (5.3)
-
Monitor renal function and potassium in susceptible patients (5.4, 5.5)
-
Exacerbation or activation of systemic lupus erythematosus (5.7)
-
Observe for signs of fluid or electrolyte imbalance (5.9)
-
Acute angle-closure glaucoma (5.10)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
Dose once-daily. The dosage may be increased after 2 weeks of therapy. The full blood pressure lowering effect was achieved 2 weeks after being on the maximal dose of Exforge HCT. The maximum recommended dose of Exforge HCT is 10/320/25 mg.
2.2 Add-on/Switch Therapy
Exforge HCT may be used for patients not adequately controlled on any 2 of the following antihypertensive classes: calcium channel blockers, angiotensin receptor blockers, and diuretics.
A patient who experiences dose-limiting adverse reactions to an individual component while on any dual combination of the components of Exforge HCT may be switched to Exforge HCT containing a lower dose of that component to achieve similar blood pressure reductions.
2.3 Replacement Therapy
Exforge HCT may be substituted for the individually titrated components.
2.4 Use With Other Antihypertensive Drugs
Exforge HCT may be administered with other antihypertensive agents.
- Dose once-daily. Titrate up to a maximum dose of 10/320/25 mg. (2.1)
- Exforge HCT may be used as add-on/switch therapy for patients not adequately controlled on any two of the following antihypertensive classes: calcium channel blockers, angiotensin receptor blockers, and diuretics. (2.2)
- Exforge HCT may be substituted for its individually titrated components. (2.3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Exforge HCT can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Published reports include cases of anhydramnios and oligohydramnios in pregnant women treated with valsartan (see Clinical Considerations).
When pregnancy is detected, discontinue Exforge HCT as soon as possible.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Valsartan
Oligohydramnios in pregnant women who use drugs affecting the renin- angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension and death.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment. Closely observe neonates with histories of in utero exposure to Exforge HCT for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to Exforge HCT, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
Hydrochlorothiazide
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma. Hydrochlorothiazide, like other diuretics, can cause placental hypoperfusion. It accumulates in the amniotic fluid, with reported concentrations up to 19 times higher than in umbilical vein plasma. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice or thrombocytopenia. Since they do not prevent or alter the course of EPH (Edema, Proteinuria, Hypertension) gestosis (preeclampsia), these drugs should not be used to treat hypertension in pregnant women. The use of HCTZ for other indications (e.g., heart disease) in pregnancy should be avoided.
Data
Animal Data
Valsartan and Amlodipine
In rats, administered 20 mg/kg/day amlodipine plus 320 mg/kg/day valsartan, treatment-related maternal and fetal effects (developmental delays and alterations noted in the presence of significant maternal toxicity) were noted with the high dose combination. This corresponds to dose multiples of 9 and 19.5 times, respectively, the maximum recommended human dose (MRHD) of 10 mg/day for amlodipine and 320 mg/day for valsartan (based on body surface area and considering a 60 kg patient).
Hydrochlorothiazide
No teratogenic effects were observed when hydrochlorothiazide was administered to mice and rats via gavage at doses of up to 3,000 and 1,000 mg/kg/day (608 and 405 times the MRHD), on gestation days 6 through 15.
8.2 Lactation
Risk Summary
There is limited information regarding the presence of Exforge HCT in human milk, the effects on the breastfed infant, or the effects on milk production. Hydrochlorothiazide is present in human milk and valsartan is present in rat milk. Limited published studies report that amlodipine is present in human milk. Because of the potential for serious adverse reactions in breastfed infants, advise a nursing woman that breastfeeding is not recommended during treatment with Exforge HCT.
Data
Valsartan was detected in the milk of lactating rats 15 minutes after oral administration of a 3 mg/kg dose.
8.4 Pediatric Use
The safety and effectiveness of Exforge HCT in pediatric patients have not been established.
8.5 Geriatric Use
Amlodipine
Clinical studies of amlodipine besylate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of AUC of approximately 40% to 60% [see Clinical Pharmacology (12.3)]. The recommended starting dose of amlodipine 2.5 mg is not an available strength with Exforge HCT [see Clinical Studies (14)].
8.6 Renal Impairment
Safety and effectiveness of Exforge HCT in patients with severe renal impairment (CrCl < 30 mL/min) have not been established. No dose adjustment is required in patients with mild (CrCl 60 to 90 mL/min) or moderate (CrCl 30 to 60 mL/min) renal impairment.
8.7 Hepatic Impairment
Amlodipine
Exposure to amlodipine is increased in patients with hepatic insufficiency. The recommended initial dose of amlodipine in patients with hepatic impairment is 2.5 mg, which is not an available strength with Exforge HCT [see Clinical Pharmacology (12.3)].
Valsartan
No dose adjustment is necessary for patients with mild-to-moderate disease. No dosing recommendations can be provided for patients with severe liver disease.
Hydrochlorothiazide
Minor alterations of fluid and electrolyte balance may precipitate hepatic coma in patients with impaired hepatic function or progressive liver disease.
Lactation: Breastfeeding is not recommended (8.2)
Geriatric Patients: Not recommended for initial therapy (8.5)
Hepatic Impairment: Not recommended for initial therapy (8.7)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Exforge HCT was studied in a double-blind, active controlled study in hypertensive patients. A total of 2271 patients with moderate to severe hypertension (mean baseline systolic/diastolic blood pressure was 170/107 mmHg) received treatments of amlodipine/valsartan/HCTZ 10/320/25 mg, valsartan/HCTZ 320/25 mg, amlodipine/valsartan 10/320 mg, or HCTZ/amlodipine 25/10 mg. At study initiation, patients assigned to the 2-component arms received lower doses of their treatment combination while patients assigned to the Exforge HCT arm received 160/12.5 mg valsartan/hydrochlorothiazide. After 1 week, Exforge HCT patients were titrated to 5/160/12.5 mg amlodipine/valsartan/hydrochlorothiazide, while all other patients continued receiving their initial doses. After 2 weeks, all patients were titrated to their full treatment dose. A total of 55% of patients were male, 14% were 65 years or older, 72% were Caucasian, and 17% were black.
At Week 8, the triple combination therapy produced greater reductions in blood pressure than each of the 3 dual combination treatments (p < 0.0001 for both diastolic and systolic blood pressures reductions). The reductions in systolic/diastolic blood pressure with Exforge HCT were 7.6/5.0 mmHg greater than with valsartan/HCTZ, 6.2/3.3 mmHg greater than with amlodipine/valsartan, and 8.2/5.3 mmHg greater than with amlodipine/HCTZ (see Figure 1). The full blood pressure lowering effect was achieved 2 weeks after being on the maximal dose of Exforge HCT (see Figure 2 and Figure 3). As the pivotal study was an active-controlled trial, the treatment effects shown in Figures 1, 2, and 3 include a placebo effect of unknown size.
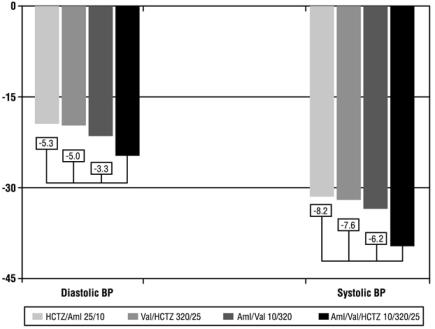
Figure 1: Reduction in Mean Blood Pressure at Endpoint
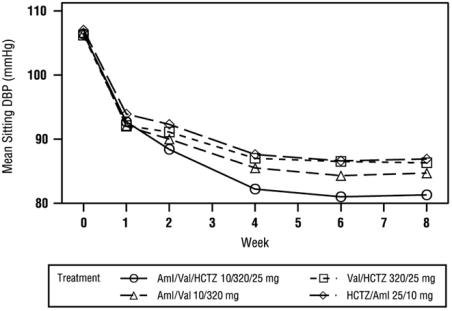
Figure 2: Mean Sitting Diastolic Blood Pressure by Treatment and Week
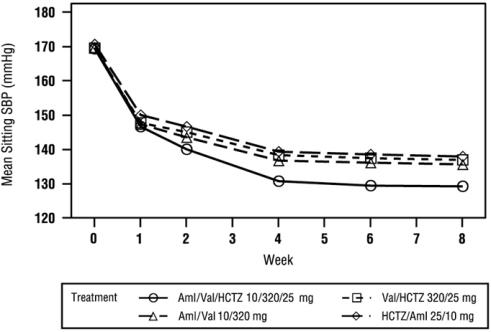
Figure 3: Mean Sitting Systolic Blood Pressure by Treatment and Week
A subgroup of 283 patients was studied with ambulatory blood pressure monitoring. The blood pressure lowering effect in the triple therapy group was maintained throughout the 24-hour period (see Figure 4 and Figure 5).
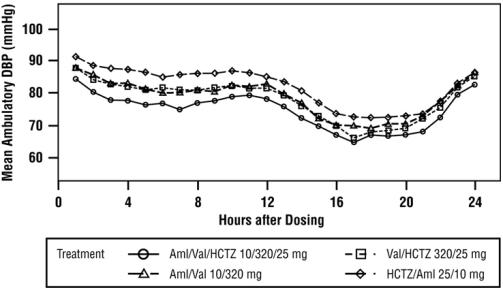
Figure 4: Mean Ambulatory Diastolic Blood Pressure at Endpoint by Treatment and Hour
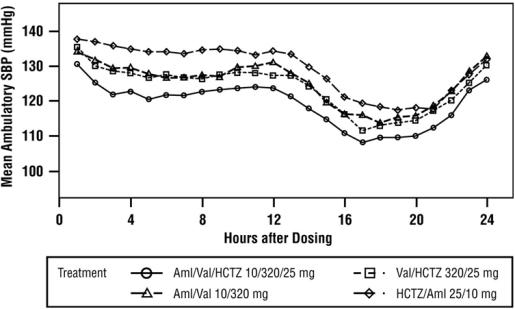
Figure 5: Mean Ambulatory Systolic Blood Pressure at Endpoint by Treatment and Hour
There are no trials of the Exforge HCT combination tablet demonstrating reductions in cardiovascular risk in patients with hypertension, but both the amlodipine and hydrochlorothiazide components and several ARBs, which are the same pharmacological class as the valsartan component, have demonstrated such benefits.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy: Advise female patients of childbearing age about the consequences of exposure to Exforge HCT during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)]
Lactation: Advise women not to breastfeed during treatment with Exforge HCT [see Use in Specific Populations (8.2)].
Symptomatic Hypotension: Advise patients that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to their healthcare provider. Tell patients that if syncope occurs to discontinue Exforge HCT until the physician has been consulted. Caution all patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope [see Warnings and Precautions (5.2)].
Potassium Supplements: Advise patients not to use salt substitutes without consulting their healthcare provider [see Drug Interactions (7)].
Non-melanoma Skin Cancer: Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening [see Adverse Reactions (6.2)].
T2021-12
