Cefepime
These highlights do not include all the information needed to use CEFEPIME Injection safely and effectively. See full prescribing information for CEFEPIME Injection. CEFEPIME injection, for intravenous useInitial U.S. Approval: 1996
be5f8ca6-7232-423a-a2d5-cccb7abe7921
HUMAN PRESCRIPTION DRUG LABEL
Jul 29, 2025
Baxter Healthcare Company
DUNS: 005083209
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
cefepime
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPLE DISPLAY PANEL
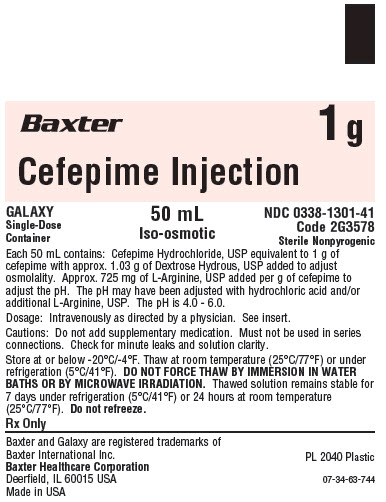
Container Label
Baxter 1 g
Cefepime Injection
GALAXY
Single-Dose
Container
50 mL
Iso-osmotic
NDC 0338-1301-41
Code 2G3578
Each 50 mL contains: Cefepime Hydrochloride, USP equivalent to 1 g of
cefepime with approx. 1.03 g of Dextrose Hydrous, USP added to adjust
osmolality. Approx. 725 mg of L-Arginine, USP added per g of cefepime to
adjust the pH. The pH may have been adjusted with hydrochloric acid and/or
additional L-Arginine, USP. The pH is 4.0 - 6.0.
Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series
connections. Check for minute leaks and solution clarity.
Store at or below -20°C/-4°F. Thaw at room temperature (25°C/77°F) or under
refrigeration (5°C/41°F).DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution remains stable for
7 days under refrigeration (5°C/41°F) or 24 hours at room temperature (25°C/77°F).Do not
** refreeze.**
Rx Only
Baxter and GALAXY are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
PL 2040 Plastic
Made in USA
07-34-63-744

Carton Label_panel 1 of 3
Thaw at room temperature (25°C/77°F) or under refrigeration (5°C/41°F).DO
NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION.
Thawed solution remains stable for 7 days under refrigeration (5°C/41°F) or 24
hours at room
temperature (25°C/77°F).Do not refreeze.
Handle frozen product containers with care. Product containers may be fragile in the frozen state.
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
07-04-65-195
Made in USA
PL 2040 Plastic
Baxter Logo
1 g
Cefepime Injection
Rx Only
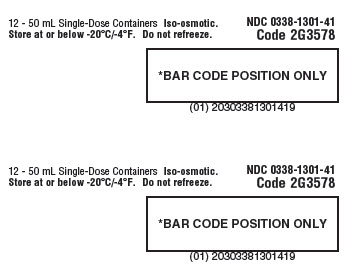
Carton Label_panel 2 of 3
12 - 50 mL Single-Dose Containers. Iso-osmotic. NDC 0338-1301-41
Store at or below -20°C/-4°F. Do not refreeze. Code 2G3578
*BAR CODE POSITION ONLY
(01) 20303381301419

Carton Label_panel 3 of 3
GALAXY Container
Sterile Nonpyrogenic
Each 50 mL contains: Cefepime Hydrochloride, USP equivalent to 1 g of cefepime with approx. 1.03 g of Dextrose Hydrous, USP added to adjust osmolality. Approx. 725 mg of L-Arginine, USP added per g of cefepime to adjust the pH. The pH may have been adjusted with hydrochloric acid and/or additional L-Arginine, USP. The pH is 4.0 - 6.0.
Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks and solution clarity. Check for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may be impaired. Do not use unless solution is clear.
DESCRIPTION SECTION
11 DESCRIPTION
Cefepime Injection in Galaxy Containers is a sterile, injectable product consisting of Cefepime Hydrochloride, USP, a semi-synthetic, broad spectrum, cephalosporin antibacterial for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-Amino-4-thiazolyl) glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-en-3-yl]methyl]-1-methylpyrrolidinium chloride, 72-(Z)-(O-methyloxime), monohydrochloride, monohydrate, which corresponds to the following structural formula:
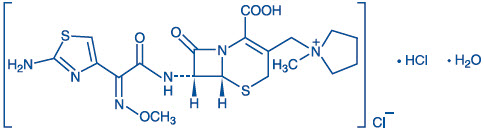
Cefepime hydrochloride (monohydrate) has a molecular mass of 571.50 and a molecular formula of C19H25ClN6O5S2•HCl•H2O.
Cefepime Injection in Galaxy Container is a frozen, iso-osmotic, sterile, non- pyrogenic premixed solution supplied for intravenous administration in strengths equivalent to 1 g and 2 g of cefepime [see Dosage and Administration (2)]. It contains the equivalent of not less than 90 percent and not more than 115 percent of the labeled amount of cefepime (C19H24N6O5S2).
The solution is intended for intravenous use after thawing to room temperature. The components and dosage formulations are given in the table below:
Table 5: Cefepime Injection in Galaxy Containers Premixed Frozen Solution|
Component* |
Function |
Dosage Formulations | |
|---|---|---|---|
|
1 g in 50 mL |
2 g in 100 mL | ||
| |||
|
Cefepime |
active ingredient |
1 g |
2 g |
|
Dextrose Hydrous, USP |
osmolality adjuster |
1.03 g |
2.06 g |
|
L-Arginine, USP† |
pH adjuster |
725 mg |
1.45 g |
|
Hydrochloric Acid† |
pH adjuster |
As needed |
As needed |
|
Water for Injection, USP |
vehicle |
q.s.‡ 50 mL |
q.s.‡ 100 mL |
Cefepime Injection will range in color from colorless to amber.
The plastic container is fabricated from a specially designed multilayer plastic. Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Adults
The recommended adult dosages and routes of administration are outlined in Table 1 below for patients with creatinine clearance greater than 60 mL/min. Administer Cefepime Injection intravenously over approximately 30 minutes.
Table 1: Recommended Dosage Schedule for Cefepime Injection in Adult Patients with Creatinine Clearance (CrCL) Greater Than 60 mL/min|
Site and Type of Infection |
Dose |
Frequency |
Duration |
|---|---|---|---|
| |||
|
Adults | |||
|
Moderate to Severe Pneumonia due to S. pneumoniae, P. aeruginosa*, K. pneumoniae, or Enterobacter species |
1-2 g IV |
Every 8-12 hours |
10 |
|
Empiric therapy for febrile neutropenic patients [see Indications and Usage (1) and Clinical Studies (14)] |
2 g IV |
Every 8 hours |
7† |
|
Mild to Moderate Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis, due to E. coli, K. pneumoniae, or P. mirabilis |
0.5-1 g IV |
Every 12 hours |
7-10 |
|
Severe Uncomplicated or Complicated Urinary Tract Infections, including pyelonephritis, due to E. coli or K. pneumoniae |
2 g IV |
Every 12 hours |
10 |
|
Moderate to Severe Uncomplicated Skin and Skin Structure Infections due to S. aureus or S. pyogenes |
2 g IV |
Every 12 hours |
10 |
|
Complicated Intra-abdominal Infections (used in combination with metronidazole) caused by E. coli, viridans group streptococci, P. aeruginosa*, K. pneumoniae, Enterobacter species, or B. fragilis. [see Clinical Studies (14)] |
2 g IV |
Every 8-12 hours |
7-10 |
2.2 Pediatric Patients (2 months up to 16 years)
The maximum dose for pediatric patients should not exceed the recommended adult dose. The usual recommended dosage in pediatric patients up to 40 kg in weight for durations as given above for adults is:
•
50 mg per kg per dose, administered every 12 hours for uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and pneumonia (see below).
•
For moderate to severe pneumonia due to P. aeruginosa give 50 mg per kg per dose, every 8 hours.
•
50 mg per kg per dose, every 8 hours for febrile neutropenic patients.
Cefepime Injection in Galaxy Container should be used only in pediatric patients who require the entire 1 or 2 g dose and not any fraction thereof.
2.3 Dosage Adjustments in Patients with Renal Impairment
Adult Patients
Adjust the dose of Cefepime Injection in patients with creatinine clearance less than or equal to 60 mL/min to compensate for the slower rate of renal elimination. In these patients, the recommended initial dose of Cefepime Injection should be the same as in patients with CrCL greater than 60 mL/min except in patients undergoing hemodialysis. The recommended doses of Cefepime Injection in patients with renal impairment are presented inTable 2.
When only serum creatinine is available, the following formula (Cockcroft and Gault equation)1 may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function:
|
Males: Creatinine Clearance (mL/min) = |
Weight (kg) x (140 – age) | |
|
72 x serum creatinine (mg/dL) | ||
|
Females: 0.85 x above value |
|
Creatinine Clearance |
Recommended Maintenance Schedule | |||
|---|---|---|---|---|
| ||||
|
Greater than 60 |
500 mg every |
1 g every |
2 g every |
2 g every |
|
30–60 |
500 mg every |
1 g every |
2 g every |
2 g every |
|
11–29 |
500 mg every |
500 mg every |
1 g every |
2 g every |
|
Less than 11 |
250 mg every |
250 mg every |
500 mg every |
1 g every |
|
Continuous |
500 mg every |
1 g every |
2 g every |
2 g every |
|
Hemodialysis* |
1 g on day 1, then 500 mg every 24 hours thereafter |
1 g every |
In patients undergoing Continuous Ambulatory Peritoneal Dialysis (CAPD), Cefepime Injection may be administered at the recommended doses at a dosage interval of every 48 hours (see Table 2).
In patients undergoing hemodialysis, approximately 68% of the total amount of cefepime present in the body at the start of dialysis will be removed during a 3-hour dialysis period. The dosage of Cefepime Injection for hemodialysis patients is 1 g on Day 1 followed by 500 mg every 24 hours for the treatment of all infections except febrile neutropenia, which is 1 g every 24 hours.
Cefepime Injection should be administered at the same time each day and following the completion of hemodialysis on hemodialysis days (see Table 2).
Pediatric Patients
Data in pediatric patients with impaired renal function are not available; however, since cefepime pharmacokinetics are similar in adults and pediatric patients [see Clinical Pharmacology (12.3)], changes in the dosing regimen proportional to those in adults (see Table 1 and Table 2) are recommended for pediatric patients.
2.4 Directions for Use of Cefepime Injection in Galaxy Container
Cefepime Injection in Galaxy Container is for intravenous administration using sterile equipment after thawing to room temperature.
Thawing of Plastic Container
Thaw frozen container at room temperature 25°C (77°F) or under refrigeration 5°C (41°F). Do not force thaw by immersion in water baths or by microwave irradiation.[See How Supplied/Storage and Handling (16).]
Check for minute leaks by squeezing container firmly. If leaks are detected, discard solution as sterility may be impaired.
Do not add supplementary medication.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Components of the solution may precipitate in the frozen state and will dissolve upon reaching room temperature with little or no agitation. Potency is not affected. Agitate after solution has reached room temperature. If after visual inspection the solution remains cloudy or if an insoluble precipitate is noted or if any seals are not intact, the container should be discarded.
Caution: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
Preparation for intravenous administration.
Suspend container from eyelet support.
Remove protector from outlet port at bottom of container.
Attach administration set. Refer to complete directions accompanying set.
Cefepime Injection should be administered intravenously over approximately 30 minutes.
Intermittent intravenous infusion with a Y-type administration set can be accomplished with compatible solutions. However, during infusion of Cefepime Injection, it is desirable to discontinue the other solution.
Solutions of cefepime, like those of most beta-lactam antibacterial drugs, should not be added to solutions of ampicillin at a concentration greater than 40 mg per mL, and should not be added to metronidazole, vancomycin, gentamicin, tobramycin, netilmicin sulfate or aminophylline because of potential interaction. However, if concurrent therapy with cefepime is indicated, each of these antibacterials can be administered separately.
As with other cephalosporins, the color of Cefepime Injection tend to darken depending on storage conditions; however, when stored as recommended, the product potency is not adversely affected.
|
Recommended Dosage in Adults With Creatinine Clearance (CrCL) | |||
|---|---|---|---|
|
Site and Type of Infection (Adults) |
Dose |
Frequency |
Duration |
| |||
|
Moderate to Severe Pneumonia* |
1-2 g |
Every |
10 |
|
Empiric therapy for febrile neutropenic patients |
2 g |
Every |
7† |
|
Mild to Moderate Uncomplicated or Complicated Urinary Tract Infections |
0.5-1 g |
Every |
7-10 |
|
Severe Uncomplicated or Complicated Urinary Tract Infections |
2 g |
Every |
10 |
|
Moderate to Severe Uncomplicated Skin and Skin Structure Infections |
2 g |
Every |
10 |
|
Complicated Intra-abdominal Infections (used in combination with metronidazole) * |
2 g |
Every |
7-10 |
•
Pediatric Patients (2 months to 16 years) – Recommended dosage in pediatric with CrCL greater than 60 mL/min. (2.2)
•
The usual recommended dosage in pediatric patients is 50 mg per kg per dose administered every 12 hours (every 8 hours for febrile neutropenia). (2.2)
•
Cefepime Injection in Galaxy Container should be used only in pediatric patients who require the entire 1 or 2 gram dose and not any fraction thereof. (2.2)
•
Patients with Renal Impairment: Adjust dose in patients with CrCL less than or equal to 60 mL/min. (2.3)
•
Administer intravenously over approximately 30 minutes. (2.1)
•
Do not force thaw frozen container by immersion in water baths or by microwave irradiation. (2.4)
