Good Sense Naproxen Sodium
Perrigo Naproxen Sodium Drug Facts
Approved
Approval ID
e5e8768d-c6e0-4018-b788-5ce86929f7b0
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Aug 26, 2025
Manufacturers
FDA
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Naproxen Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code71335-2584
Application NumberANDA074661
Product Classification
M
Marketing Category
C73584
G
Generic Name
Naproxen Sodium
Product Specifications
Route of AdministrationORAL
Effective DateAugust 26, 2025
FDA Product Classification
INGREDIENTS (9)
NAPROXEN SODIUMActive
Quantity: 220 mg in 1 1
Code: 9TN87S3A3C
Classification: ACTIB
FD&C BLUE NO. 2Inactive
Code: L06K8R7DQK
Classification: IACT
HYPROMELLOSE, UNSPECIFIEDInactive
Code: 3NXW29V3WO
Classification: IACT
POLYETHYLENE GLYCOL, UNSPECIFIEDInactive
Code: 3WJQ0SDW1A
Classification: IACT
POVIDONE, UNSPECIFIEDInactive
Code: FZ989GH94E
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
MICROCRYSTALLINE CELLULOSEInactive
Code: OP1R32D61U
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
Drug Labeling Information
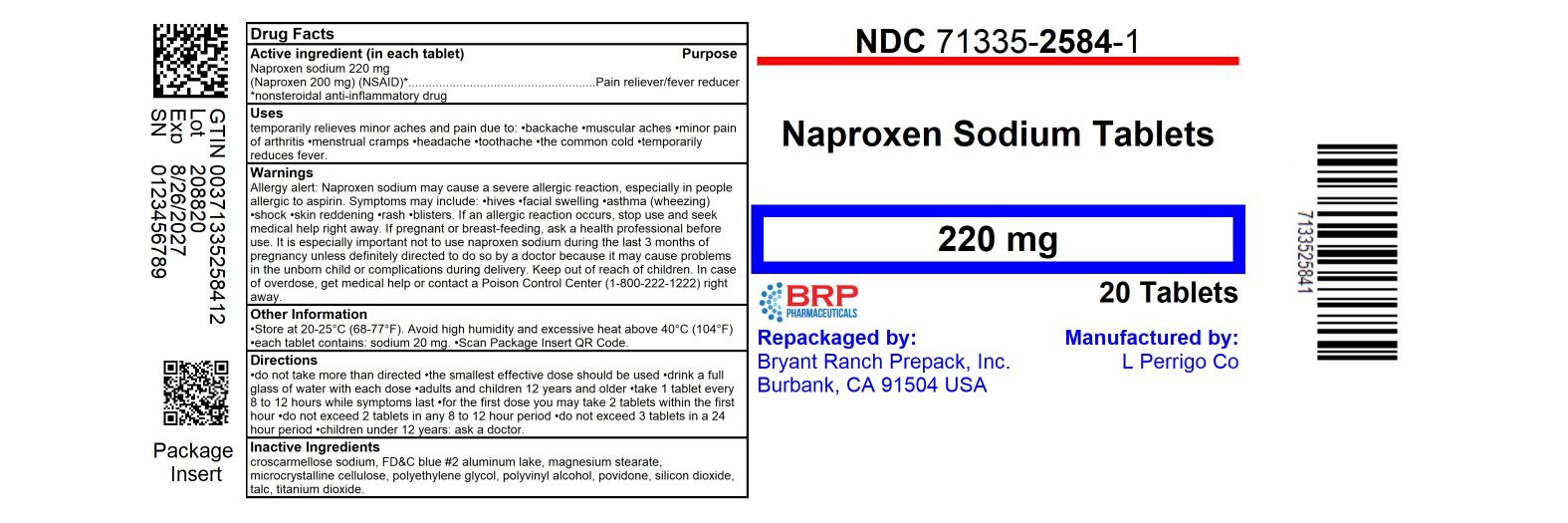
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 4/8/2025
Naproxen Sodium 220mg Tablet
How Supplied Section
LOINC: 34069-5Updated: 8/26/2025
HOW SUPPLIED
Naproxen Sodium Tablets 220mg - Blue (Light Blue), Oval, Imprint "L368".
- NDC 71335-2584-1: 20 Tablets in a BOTTLE
- NDC 71335-2584-2: 30 Tablets in a BOTTLE
- NDC 71335-2584-3: 40 Tablets in a BOTTLE
- NDC 71335-2584-4: 50 Tablets in a BOTTLE
- NDC 71335-2584-5: 60 Tablets in a BOTTLE
- NDC 71335-2584-6: 14 Tablets in a BOTTLE
- NDC 71335-2584-7: 100 Tablets in a BOTTLE
- NDC 71335-2584-8: 90 Tablets in a BOTTLE
- NDC 71335-2584-9: 24 Tablets in a BOTTLE
Store at 20-25°C (68-77°F). Avoid high humidity and excessive heat above 40°C (104°F).
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
