GAVILYTE-N
These highlights do not include all the information needed to use GaviLyte – N safely and effectively. See full prescribing information for GaviLyte – N GaviLyte™ – N (polyethylene glycol 3350 (420 g), sodium chloride, sodium bicarbonate and potassium chloride for oral solution)Initial U.S. Approval: 1991
27195187-75a3-431f-90d9-84da1b94e849
HUMAN PRESCRIPTION DRUG LABEL
Sep 10, 2025
Lupin Pharmaceuticals,Inc.
DUNS: 089153071
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
polyethylene glycol-3350, sodium chloride, potassium chloride and sodium bicarbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
GaviLyte-N
Container Label

Flavor Packs (Cherry Flavor, Lemon Flavor and Orange Flavor)

DESCRIPTION SECTION
11 DESCRIPTION
For oral solution: Each 4 liter (4L) GaviLyte-N jug contains a white powder for reconstitution. GaviLyte-N is a combination of polyethylene glycol 3350, an osmotic laxative, and electrolytes (sodium chloride, sodium bicarbonate and potassium chloride) for oral solution.
Each 4 liter jug contains: polyethylene glycol 3350 420g, sodium bicarbonate 5.72 g, sodium chloride 11.2 g, potassium chloride 1.4 g. The solution is clear and colorless when reconstituted to a final volume of 4 liters with water.
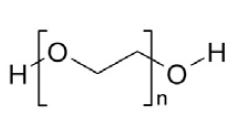
Polyethylene Glycol 3350, NF
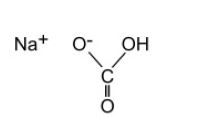
Sodium Bicarbonate, USP
The chemical name is NaHCO3. The average Molecular Weight is 84.01. The structural formula is:
Sodium Chloride, USP
The chemical name is NaCl. The average Molecular Weight: 58.44. The structural formula is:
Na+ Cl-
Potassium Chloride, USP
The chemical name is KCl. The average Molecular Weight: 74.55. The structural formula is:
K-Cl
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Medication Guide). Instruct patients:
- To let you know if they have trouble swallowing or are prone to regurgitation or aspiration.
- Not to take other laxatives while they are taking GaviLyte-N.
- To consume water or clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- That if they experience severe bloating, distention or abdominal pain, the administration of the solution should be slowed or temporarily discontinued until the symptoms abate. Advise patients to report these events to their health care provider.
- That if they have hives, rashes, or any allergic reaction, they should discontinue the medication and contact their health care provider. Medication should be discontinued until they speak to their physician.
- To contact their healthcare provider if they develop signs and symptoms of dehydration [see Warnings and Precautions (5.1)].
- That oral medication administered within one hour of the start of administration of GaviLyte-N solution may be flushed from the GI tract and the medication may not be absorbed completely.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples FL, 34108
SAP Code: 276329
Rev. 11/2024
SPL MEDGUIDE SECTION
MEDICATION GUIDE
GaviLyte™- N (GAV-ee-LITE-N)
(polyethylene glycol 3350 (420 g), sodium chloride, sodium bicarbonate and potassium chloride oral solution)
Read this Medication Guide before you start taking GaviLyte-N. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about GaviLyte-N?
GaviLyte-N and other osmotic bowel preparations can cause serious side effects, including:
Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
These changes can cause:
*abnormal heartbeats that can cause death *seizures . This can happen even if you have never had a seizure. *kidney problems
Your chance of having fluid loss and changes in body salts with GaviLyte-N is higher if you:
- have heart problems
- have kidney problems
- take water pills or non-steroidal anti-inflammatory drugs (NSAIDS)
Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking GaviLyte-N:
- vomiting that prevents you from keeping down the solution
- dizziness
- urinating less often than normal
- headache
See Section "What are the possible side effects of GaviLyte-N" for more information about side effects.
What is GaviLyte-N?
GaviLyte-N is a prescription medicine used by adults to clean the colon before a colonoscopy. GaviLyte-N cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
GaviLyte-N is safe and effective for use in pediatric patients aged 6 months and older.
Who should not take GaviLyte-N?
Do not take GaviLyte-N if your healthcare provider has told you that you have:
- a blockage in your bowel (obstruction)
- an opening in the wall of your stomach or intestine (bowel perforation)
- problems with food and fluid emptying from your stomach (gastric retention)
- a very dilated intestine (bowel)
- an allergy to any of the ingredients in GaviLyte-N. See the end of this leaflet for a complete list of ingredients in GaviLyte-N.
What should I tell my healthcare provider before taking GaviLyte-N?
Before you take GaviLyte-N, tell your healthcare provider if you:
- have heart problems
- have stomach or bowel problems
- have ulcerative colitis
- have problems with swallowing or gastric reflux
- have a history of seizures
- are withdrawing from drinking alcohol
- have a low blood salt (sodium) level
- have kidney problems
- any other medical conditions
- are pregnant. It is not known if GaviLyte-N will harm your unborn baby.
Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if GaviLyte-N passes into your breast milk. You and your healthcare provider should decide if you will take GaviLyte-N while breastfeeding.
**Tell your healthcare provider about all the medicines you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements.
GaviLyte-N may affect how other medicines work. Medicines taken by mouth may not be absorbed properly when taken within 1 hour before the start of GaviLyte-N.
Especially tell your healthcare provider if you take:
- medicines for blood pressure or heart problems
- medicines for kidney problems
- medicines for seizures
- water pills (diuretics)
- non-steroidal anti-inflammatory medicines (NSAID) pain medicines
- laxatives
- starch-based thickeners. For patients who have trouble swallowing, do not mix GaviLyte-N with starch-based thickeners.
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take GaviLyte-N?
You must read, understand, and follow these instructions to take GaviLyte-N the right way.
- Take GaviLyte-N exactly as your healthcare provider tells you to take it.
- Drink 240 mL (8 oz.) every 10 minutes. Rapid drinking of each portion is better than drinking small amounts.
- The first bowel movement should occur approximately one hour after you start drinking the solution.
- You may experience some abdominal bloating and distention before the bowels start to move. If severe discomfort or distention occur, stop drinking temporarily or drink each portion at longer intervals until the discomfort goes away.
- Continue drinking until the watery stool is clear and free of solid matter. This usually requires 3 liters and it is best to drink all of the solution. *Do not take undissolved GaviLyte-N powder that has not been mixed with water (diluted), it may increase your risk of nausea, vomiting and fluid loss (dehydration).
- Each jug of GaviLyte-N must be reconstituted with water (diluted) to 4 liters total volume before drinking.
- Do not take other laxatives while taking GaviLyte-N.
*Do not eat solid foods on the day before your colonoscopy and until after your colonoscopy . Drink only clear liquids:
- the day before your colonoscopy
- while taking GaviLyte-N
- after taking GaviLyte-N until 2 hours before your colonoscopy
What are the possible side effects of GaviLyte-N?
GaviLyte-N can cause serious side effects, including:
*See Section "What is the most important information I should know about GaviLyte-N?" *changes in certain blood tests . Your healthcare provider may do blood tests after you take GaviLyte-N to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including: * vomiting * nausea * bloating * dizziness * stomach (abdominal) cramping * headache * urinate less than usual * trouble drinking clear liquid
*heart problems. GaviLyte-N may cause irregular heartbeats. *seizures *ulcers of the bowel or bowel problems (ischemic colitis). Tell your healthcare provider right away if you have severe stomach-area (abdomen) pain or rectal bleeding.
The most common side effects of GaviLyte-N include:
- nausea
- stomach (abdominal) fullness
- bloating
- stomach (abdominal) cramps
- vomiting
- anal irritation
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of GaviLyte-N. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GaviLyte-N?
- Store GaviLyte-N at room temperature, between 59ºF to 86°F (15ºC to 30°C).
Keep GaviLyte-N and all medicines out of the reach of children.
General information about the safe and effective use of GaviLyte-N.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GaviLyte-N for a condition for which it was not prescribed. Do not give GaviLyte-N to other people, even if they are going to have the same procedure you are. It may harm them.
This Medication Guide summarizes important information about GaviLyte-N. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
For more information, call 1-866-403-7592.
What are the ingredients in GaviLyte-N?
Active ingredients**:** polyethylene glycol 3350, sodium bicarbonate, sodium chloride, and potassium chloride.
Inactive ingredients**:** cherry flavoring, lemon flavoring, orange flavoring (flavor packs only)
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples FL, 34108
This Medication Guide has been approved by the U.S. Food and Drug Administration.
SAP Code: 276329
Rev. 11/2024
