AZSTARYS
These highlights do not include all the information needed to use AZSTARYS safely and effectively. See full prescribing information for AZSTARYS. AZSTARYS (serdexmethylphenidate and dexmethylphenidate) capsules, for oral use, CII Initial U.S. Approval: 2021
00b5e716-5564-4bbd-acaf-df2bc45a5663
HUMAN PRESCRIPTION DRUG LABEL
Oct 18, 2023
Corium, LLC.
DUNS: 839674207
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Serdexmethylphenidate and Dexmethylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Serdexmethylphenidate and Dexmethylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Serdexmethylphenidate and Dexmethylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC: 65038-561-99 - 52.3/10.4 mg 100 count Bottle
Label
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
AZSTARYS has a high potential for abuse and misuse. The use of AZSTARYS exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. AZSTARYS can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including AZSTARYS, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing AZSTARYS, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store AZSTARYS in a safe place, preferably locked, and instruct patients to not give AZSTARYS to anyone else. Throughout AZSTARYS treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid AZSTARYS use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 beats per minute). Some patients may have larger increases.
Monitor all AZSTARYS-treated patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-Existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed mood episode in patients. Prior to initiating AZSTARYS treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosage, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing AZSTARYS.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use, in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on methylphenidate , often subsequent to an increase in dosage. Priapism also occurred during methylphenidate withdrawal (drug holidays or during discontinuation).
AZSTARYS-treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, including Raynaud's Phenomenon
CNS stimulants used to treat ADHD, including AZSTARYS, are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post- marketing reports and at the therapeutic dosage of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during AZSTARYS treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for AZSTARYS-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.7 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
In a long-term, open-label safety study with AZSTARYS conducted in pediatric patients 6 to 12 years of age with ADHD, there was a lower than expected increase in height and weight compared to pediatric patients of the same age and sex, on average [see Adverse Reactions (6.1)].
Closely monitor growth (weight and height) in AZSTARYS-treated pediatric patients. Pediatric patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.8 Acute Angle Closure Glaucoma
There have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, AZSTARYS-treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
5.9 Increased Intraocular Pressure and Glaucoma
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment [see Adverse Reactions (6.2)].
Prescribe AZSTARYS to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor AZSTARYS-treated patients with a history of abnormally increased IOP or open angle glaucoma.
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating AZSTARYS, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor AZSTARYS-treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
- Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease. (5.2)
- Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse. (5.3)
- Psychiatric Adverse Reactions: Prior to initiating AZSTARYS, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing AZSTARYS. (5.4)
- Priapism: If abnormally sustained or frequent and painful erections occur, patients should seek immediate medical attention. (5.5)
- Peripheral Vasculopathy, including Raynaud's Phenomenon: Careful observation for digital changes is necessary during AZSTARYS treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy. (5.6)
- Long-Term Suppression of Growth in Pediatric Patients: Monitor height and weight at appropriate intervals in pediatric patients. (5.7)
- Acute Angle Closure Glaucoma: AZSTARYS-treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist. (5.8)
- Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe AZSTARYS to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor patients with a history of increased IOP or open angle glaucoma. (5.9)
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome: Before initiating AZSTARYS, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette’s syndrome. Discontinue treatment if clinically appropriate. (5.10)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Known hypersensitivity to methylphenidate or other ingredients of AZSTARYS [see Contraindications (4)]
- Hypertensive Crisis with Concomitant Use of Monoamine Oxidase Inhibitors [see Contraindications (4)]
- Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, including Raynaud's Phenomenon [see Warnings and Precautions (5.6)]
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.7)]
- Acute Angle Closure Glaucoma [see Warnings and Precautions (5.8)]
- Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions (5.9)]
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Studies with Other Methylphenidate Products in Pediatric Patients and Adults with ADHD
Commonly reported (≥ 5% of the methylphenidate group and at least twice the rate of the placebo group) adverse reactions from placebo-controlled trials of methylphenidate products include: decreased appetite, decreased weight, nausea, abdominal pain, dyspepsia, vomiting, insomnia, anxiety, affect lability, irritability, dizziness, increased blood pressure, and tachycardia.
Adverse Reactions in Studies with AZSTARYS in Pediatric Patients (6 to 12 years) with ADHD
Short-Term Study
A short-term study conducted in pediatric patients 6 to 12 years of age with ADHD was comprised of a 3-week, open-label, dose optimization phase in which all patients received AZSTARYS (n=155), followed by a 1-week, double-blind, controlled phase in which patients were randomized to continue AZSTARYS (n=74) or switch to placebo (n=76). Because of the study design, the reported adverse reaction rates cannot be used to predict the rates that may be expected in clinical practice.
Long-Term Study
A long-term, open-label safety study was conducted in pediatric patients 6 to 12 years of age with ADHD who either completed the short-term study or were de novo patients. This study was comprised of a 3-week dose optimization phase for patients not recently treated with AZSTARYS followed by a 12-month treatment phase for all patients during which 238 patients received open- label AZSTARYS and had evaluable safety data. A total of 154 patients were treated for 12 months. Because of the open-label, uncontrolled design of this study, the reported adverse reaction rates cannot be assessed in terms of a causal relationship to AZSTARYS treatment.
To adjust for normal growth, z-scores were derived (measured in standard deviations [SD]); z- scores normalize for the natural growth of children and adolescents by comparisons to age- and sex-matched population standards. A z-score change less than 0.5 SD is considered not clinically significant.
In this study, the mean increase in weight from baseline to Month 12 was 3.4 kg among study completers. The mean change in z-score from baseline to Month 12 was -0.20, indicating a lower than expected increase in body weight compared to children of the same age and sex, on average. Most of the weight z-score decline occurred in the first 4 months of treatment.
The mean increase in height from baseline to Month 12 was 4.9 cm among completers. Using the same z-score analysis for height, the mean change in z-score from baseline to Month 12 was - 0.21, indicating a lower than expected increase in height compared to pediatric patients of the same age and sex, on average.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of methylphenidate products. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are as follows:
Blood and Lymphatic System Disorders: pancytopenia, thrombocytopenia, thrombocytopenic purpura
Cardiac Disorders: angina pectoris, bradycardia, extrasystole, supraventricular tachycardia, ventricular extrasystole, palpitations, increased heart rate
Eye Disorders: diplopia, increased intraocular pressure, mydriasis, visual impairment, blurred vision
General Disorders: chest pain, chest discomfort, hyperpyrexia
Gastrointestinal Disorders: dry mouth
Hepatobiliary disorders: hepatocellular injury, acute hepatic failure
Immune System Disorders: hypersensitivity reactions such as angioedema, anaphylactic reactions, auricular swelling, bullous conditions, exfoliative conditions, urticarias, pruritus NEC, rashes, eruptions, and exanthemas NEC
Investigations: alkaline phosphatase increased, bilirubin increased, hepatic enzyme increased, platelet count decreased, white blood cell count abnormal
Musculoskeletal, Connective Tissue and Bone Disorders: arthralgia, myalgia, muscle twitching, rhabdomyolysis, muscle cramps
Nervous System: convulsion, grand mal convulsion, dyskinesia, serotonin syndrome in combination with serotonergic drugs, nervousness, headache, tremor, drowsiness, vertigo, motor and verbal tics
Psychiatric Disorders: disorientation, libido changes, hallucination, hallucination auditory, hallucination visual, logorrhea, mania, restlessness, agitation
Skin and Subcutaneous Tissue Disorders: alopecia, erythema, hyperhidrosis
Urogenital System: priapism
Vascular Disorders: Raynaud's phenomenon
Based on accumulated data from other methylphenidate products, the most common (>5% and twice the rate of placebo) adverse reactions are appetite decreased, insomnia, nausea, vomiting, dyspepsia, abdominal pain, weight decreased, anxiety, dizziness, irritability, affect lability, tachycardia, and blood pressure increased. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Corium, Inc. at 1-800-910-8432 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE SECTION
10 OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of AZSTARYS should be considered when treating patients with overdose. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime studies to evaluate the carcinogenic potential of serdexmethylphenidate have not been conducted.
Lifetime carcinogenicity studies have not been carried out with dexmethylphenidate hydrochloride.
In a lifetime carcinogenicity study carried out in B6C3F1 mice, racemic methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas was seen at a daily dose of approximately 60 mg/kg/day. This dose is approximately 4 times the MRHD of 40 mg of dexmethylphenidate hydrochloride on a mg/m2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Racemic methylphenidate hydrochloride did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 5 times the MRHD of 40 mg of dexmethylphenidate hydrochloride on a mg/m2 basis.
In a 24-week carcinogenicity study with racemic methylphenidate in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentrations as in the lifetime carcinogenicity study; the high-dose group was exposed to 60 to 74 mg/kg/day of racemic methylphenidate hydrochloride.
Mutagenesis
Serdexmethylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitro mammalian cell micronucleus assay using human peripheral blood lymphocytes, in the in vivo rat bone barrow micronucleus assay, or in the in vivo rat alkaline comet assay.
Dexmethylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitro mouse lymphoma cell forward mutation assay, or in the in vivo mouse bone marrow micronucleus test. In an in vitro assay using cultured Chinese Hamster Ovary (CHO) cells treated with racemic methylphenidate, sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response.
Impairment of Fertility
Racemic methylphenidate hydrochloride did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week continuous breeding study. The study was conducted at doses of up to 160 mg/kg/day, approximately 10-times the MRHD of 40 mg of dexmethylphenidate hydrochloride on a mg/m2 basis.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Pediatric Patients 6 to 12 years of age with ADHD
The efficacy of AZSTARYS for the treatment of ADHD in pediatric patients 6 to 12 years of age was evaluated in a randomized, double-blind, placebo- controlled, parallel group, analog classroom study (Study 1; NCT# 03292952). That study was conducted in 150 pediatric patients 6 to 12 years of age who met Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria for a primary diagnosis of ADHD (combined, inattentive, or hyperactive/impulsive presentation) confirmed by the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID).
Following washout of previous ADHD medication, subjects entered an open-label dose- optimization period (3 weeks) with an initial dosage of 39.2 mg/7.8 mg once daily in the morning. The dose could be titrated on a weekly basis to either 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg, until an optimal dose or the maximum dosage of 52.3 mg/10.4 mg/day was reached. At the end of optimization period, subjects were randomly assigned into a 1-week parallel group treatment period to receive either the individually optimized dose of AZSTARYS (mean dose of 45.6 mg/9.0 mg) or placebo.
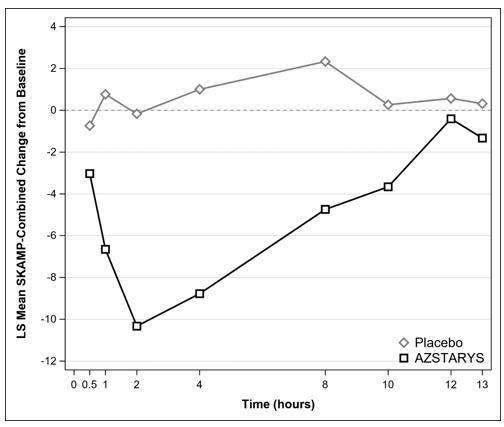
At the end of the 1-week treatment period, raters evaluated the attention and behavior of the subjects in a laboratory classroom setting over a period of 13 hours using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale. SKAMP is a validated 13-item teacher-rated scale that assesses manifestations of ADHD in a classroom setting. On this day, the dose was administered in the morning immediately after breakfast.
The primary efficacy endpoint was the mean change from baseline (pre-dose at randomization visit) of the SKAMP-Combined scores averaged across the test day (not including baseline score), with assessments conducted at 0.5, 1, 2, 4, 8, 10, 12, and 13 hours post-dose.
The mean change from baseline in the SKAMP-Combined scores, averaged across the test day, was statistically significantly lower (indicating improvement) with AZSTARYS compared to placebo (Table 2).
Table 2: Primary Efficacy Measure: SKAMP-Combined Scores Averaged Over Classroom Day in Pediatric Patients (6 to 12 years) with ADHD|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
| |||||
|
Study Number |
Treatment Group |
N |
Mean Baseline Score (SD)* |
LS Mean Change from Baseline† (SE) |
Placebo-subtracted Difference‡ (95% CI) |
|
Study 1 |
AZSTARYS (26.1 /5.2, 39.2/7.8, 52.3/10.4 mg/day) |
74 |
17.9 (9.2) |
-4.87 (0.62) |
-5.4 |
|
Placebo |
76 |
17.9 (10.4) |
0.54 (0.70) |
Figure 2: LS Mean Change in SKAMP-Combined Score from Baseline after Treatment with AZSTARYS or Placebo during Classroom Day in Pediatric Patients (6 to 12 years) with ADHD
Adults and Pediatric Patients 13 to 17 years of age with ADHD
The efficacy of 52.3 mg/10.4 mg AZSTARYS in adults and pediatric patients 13 to 17 years of age was established by pharmacokinetic bridging between AZSTARYS (52.3 mg/10.4 mg) and dexmethylphenidate hydrochloride extended- release capsules [see Clinical Pharmacology (12.3)].
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 10/2023 | ||
|
Medication Guide | |||
|
What is the most important information I should know about AZSTARYS? | |||
|
AZSTARYS can cause serious side effects, including: Abuse***misuse, and addiction.****AZSTARYS has a high chance for abuse and misuse and may lead to substance use problems, including addiction. Misuse and abuse of AZSTARYS, other methylphenidate containing medicines, and amphetamine containing medicines, can lead to overdose and death. The risk of overdose and death is increased with higher doses of AZSTARYS or when it is used in ways that are not approved, such as snorting or injection.
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with AZSTARYS. Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects. Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with AZSTARYS. *Increased blood pressure and heart rate. Your healthcare provider should check you or your child’s blood pressure and heart rate regularly during treatment with AZSTARYS. *Mental (psychiatric) problems, including: * new or worse behavior and thought problems * new or worse bipolar illness * new psychotic symptoms (such as hearing voices, or seeing or believing things that are not real) or new manic symptoms Tell your healthcare provider about any mental problems you or your child
have, or about a family history of suicide, bipolar illness, or depression. | |||
|
What is AZSTARYS? | |||
|
AZSTARYS is a central nervous system stimulant prescription medicine used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in people 6 years of age and older. AZSTARYS may help increase attention and decrease impulsiveness and hyperactivity in people 6 years of age and older with ADHD. | |||
|
It is not known if AZSTARYS is safe and effective in children younger than 6 years of age. | |||
|
AZSTARYS is a federally controlled substance (CII) because it contains dexmethylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep AZSTARYS in a safe place to protect it from theft. Never give AZSTARYS to anyone else because it may cause death or harm them. Selling or giving away AZSTARYS may harm others and is against the law. | |||
|
Do not take AZSTARYS if you or your child are:
| |||
|
Before taking AZSTARYS, tell your healthcare provider about all medical conditions, including if you or your child:
| |||
|
Tell your healthcare provider about all of the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||
|
AZSTARYS and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted during treatment with AZSTARYS. Your healthcare provider will decide whether AZSTARYS can be taken with other medicines. | |||
|
**Especially tell your healthcare provider if you or your child takes: **blood pressure medicines (anti-hypertensive) | |||
|
Know the medicines that you or your child take. Keep a list of the medicines with you to show your healthcare provider and pharmacist.Do not start any new medicine during treatment with AZSTARYS without talking to your healthcare provider first. | |||
|
How should AZSTARYS be taken?
If you or your child take too much AZSTARYS, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away. | |||
|
What are possible side effects of AZSTARYS? | |||
|
AZSTARYS can cause serious side effects, including:
*Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment AZSTARYS. AZSTARYS treatment may be stopped if your child is not growing or gaining weight. ***Eye problems (increased pressure in the eye and glaucoma).**Call your healthcare provider right away if you or your child develop changes in your vision or eye pain, swelling, or redness. ***New or worsening tics or worsening Tourette’s syndrome.**Tell your healthcare provider if you or your child get any new or worsening tics or worsening Tourette’s syndrome during treatment with AZSTARYS. | |||
|
The most common side effects of AZSTARYS include: | |||
|
| ||
|
These are not all the possible side effects of AZSTARYS. | |||
|
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
How should I store AZSTARYS?
| |||
|
Keep AZSTARYS and all medicines out of the reach of children. | |||
|
General information about the safe and effective use of AZSTARYS. | |||
|
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use AZSTARYS for a condition for which it was not prescribed. Do not give AZSTARYS to other people, even if they have the same symptoms that you have. It may harm them, and it is against the law. You can ask your healthcare provider or pharmacist for information about AZSTARYS that was written for healthcare professionals. | |||
|
What are the ingredients in AZSTARYS? | |||
|
Active ingredients: serdexmethylphenidate and dexmethylphenidate | |||
|
Inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, talc, black iron oxide, titanium dioxide, FD&C Blue No. 1 (26/5.2 mg and 39/7.8 mg), FD&C Red No. 40 (39/7.8 mg and 52/10.4 mg), FD&C Yellow No. 6 (52/10.4 mg) | |||
|
Distributed by Corium, LLC, 11 Farnsworth St., 4th Floor , Boston, MA 02210 | |||
|
For more information call 1-800-910-8432 or go to www.AZSTARYS.com |
