Rosuvastatin
These highlights do not include all the information needed to use ROSUVASTATIN TABLETS safely and effectively. See full prescribing information for ROSUVASTATIN TABLETS. ROSUVASTATIN tablets, for oral use Initial U.S. Approval: 2003
979588e5-d266-4728-aa58-36cd6a217584
HUMAN PRESCRIPTION DRUG LABEL
Oct 5, 2023
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rosuvastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
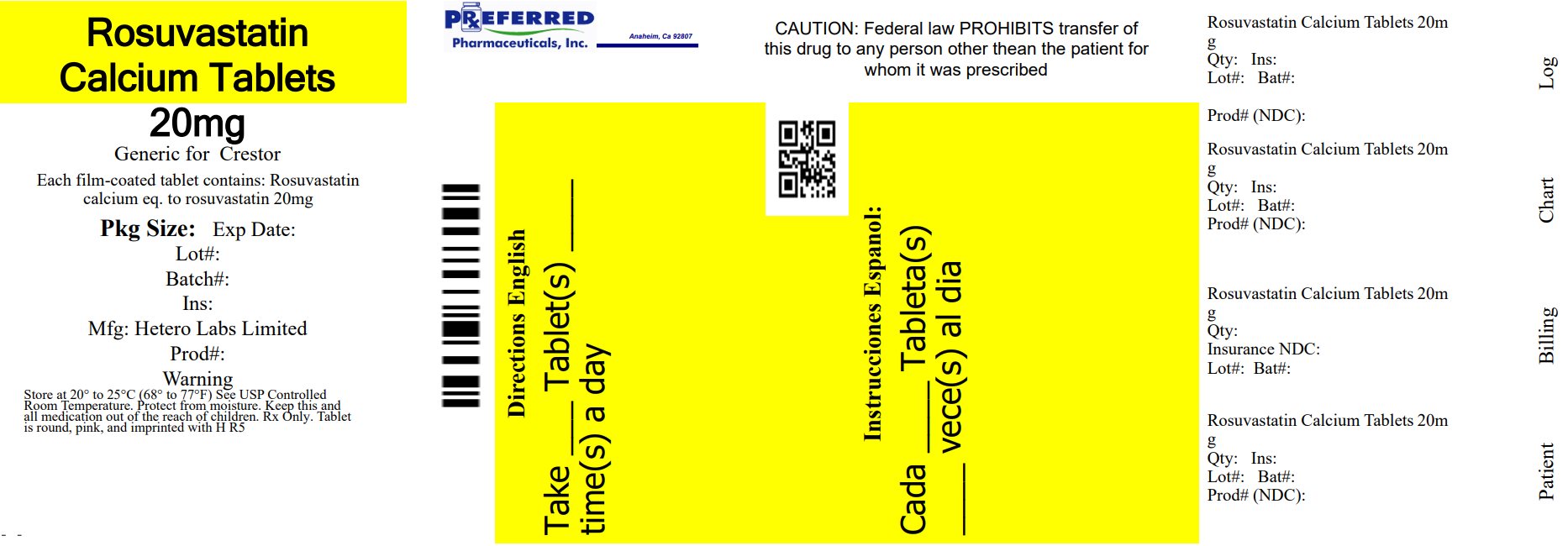
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
20 mg
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca's CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
1.3 Hypertriglyceridemia
Rosuvastatin tablets are indicated as adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia.
1.4 Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia)
Rosuvastatin tablets are indicated as an adjunct to diet for the treatment of adult patients with primary dysbetalipoproteinemia (Type III Hyperlipoproteinemia).
1.5 Adult Patients with Homozygous Familial Hypercholesterolemia
Rosuvastatin tablets are indicated as adjunctive therapy to other lipid- lowering treatments (e.g., LDL apheresis) or alone if such treatments are unavailable to reduce LDL-C, Total-C, and ApoB in adult patients with homozygous familial hypercholesterolemia.
1.8 Limitations of Use
Rosuvastatin tablets have not been studied in Fredrickson Type I and V dyslipidemias.
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to
myoglobinuria have been reported with HMG-CoA reductase inhibitors, including
rosuvastatin. These risks can occur at any dose level, but are increased at
the highest dose (40 mg).
Rosuvastatin should be prescribed with caution in patients with predisposing
factors for myopathy (e.g., age ≥65 years, inadequately treated
hypothyroidism, renal impairment).
The risk of myopathy during treatment with rosuvastatin may be increased with
concurrent administration of some other lipid-lowering therapies (fibrates or
niacin), gemfibrozil, cyclosporine, atazanavir/ritonavir, lopinavir/ritonavir,
or simeprevir [see Dosage and Administration (2) and Drug Interactions (7)].
Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA
reductase inhibitors, including rosuvastatin, coadministered with colchicine,
and caution should be exercised when prescribing rosuvastatin with colchicine
[see Drug Interactions (7.7)].
Rosuvastatin therapy should be discontinued if markedly elevated creatine
kinase levels occur or myopathy is diagnosed or suspected. Rosuvastatin
therapy should also be temporarily withheld in any patient with an acute,
serious condition suggestive of myopathy or predisposing to the development of
renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension,
dehydration, major surgery, trauma, severe metabolic, endocrine, and
electrolyte disorders, or uncontrolled seizures).
There have been rare reports of immune-mediated necrotizing myopathy (IMNM),
an autoimmune myopathy, associated with statin use. IMNM is characterized by:
proximal muscle weakness and elevated serum creatine kinase, which persist
despite discontinuation of statin treatment; muscle biopsy showing necrotizing
myopathy without significant inflammation; improvement with immunosuppressive
agents.
All patients should be advised to promptly report to their physician
unexplained muscle pain, tenderness, or weakness, particularly if accompanied
by malaise or fever or if muscle signs and symptoms persist after
discontinuing rosuvastatin.
5.2 Liver Enzyme Abnormalities
It is recommended that liver enzyme tests be performed before the initiation
of rosuvastatin, and if signs or symptoms of liver injury occur.
Increases in serum transaminases [AST (SGOT) or ALT (SGPT)] have been reported
with HMG-CoA reductase inhibitors, including rosuvastatin. In most cases, the
elevations were transient and resolved or improved on continued therapy or
after a brief interruption in therapy. There were two cases of jaundice, for
which a relationship to rosuvastatin therapy could not be determined, which
resolved after discontinuation of therapy. There were no cases of liver
failure or irreversible liver disease in these trials.
In a pooled analysis of placebo-controlled trials, increases in serum
transaminases to >3 times the upper limit of normal occurred in 1.1% of
patients taking rosuvastatin versus 0.5% of patients treated with placebo.
There have been rare postmarketing reports of fatal and non-fatal hepatic
failure in patients taking statins, including rosuvastatin. If serious liver
injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs
during treatment with rosuvastatin, promptly interrupt therapy. If an
alternate etiology is not found, do not restart rosuvastatin.
Rosuvastatin should be used with caution in patients who consume substantial
quantities of alcohol and/or have a history of chronic liver disease [see Clinical Pharmacology (12.3)]. Active liver disease, which may include
unexplained persistent transaminase elevations, is a contraindication to the
use of rosuvastatin [see Contraindications (4)].
5.3 Concomitant Coumarin Anticoagulants
Caution should be exercised when anticoagulants are given in conjunction with rosuvastatin because of its potentiation of the effect of coumarin-type anticoagulants in prolonging the prothrombin time/INR. In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs [see Drug Interactions (7.4)].
5.4 Proteinuria and Hematuria
In the rosuvastatin clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin treated patients. These findings were more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator HMG-CoA reductase inhibitors, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, a dose reduction should be considered for patients on rosuvastatin therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
5.5 Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with
HMG-CoA reductase inhibitors, including rosuvastatin. Based on clinical trial
data with rosuvastatin, in some instances these increases may exceed the
threshold for the diagnosis of diabetes mellitus [see Adverse Reactions (6.1)].
Although clinical studies have shown that rosuvastatin alone does not reduce
basal plasma cortisol concentration or impair adrenal reserve, caution should
be exercised if rosuvastatin is administered concomitantly with drugs that may
decrease the levels or activity of endogenous steroid hormones such as
ketoconazole, spironolactone, and cimetidine.
5.6 Risk of Allergic Reactions due to Tartrazine
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic- type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
•** Skeletal muscle effects (e.g., myopathy and rhabdomyolysis):** Risks
increase with use of 40 mg dose, advanced age (≥65), hypothyroidism, renal
impairment, and combination use with cyclosporine, atazanavir/ritonavir,
lopinavir/ritonavir, or simeprevir. Cases of myopathy and rhabdomyolysis with
acute renal failure secondary to myoglobinuria have been reported. Advise
patients to promptly report to their physician unexplained and/or persistent
muscle pain, tenderness, or weakness and discontinue rosuvastatin if signs or
symptoms appear. (5.1, 7.5, 7.6)
•Liver enzyme abnormalities: Persistent elevations in hepatic
transaminases can occur. Perform liver enzyme tests before initiating therapy
and as clinically indicated thereafter. (5.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in
other sections of the label:
• Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy
(including myositis) [see Warnings and Precautions (5.1)]
• Liver enzyme abnormalities [see Warnings and Precautions (5.2)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions,
adverse reaction rates observed in the clinical studies of a drug cannot be
directly compared to rates in the clinical studies of another drug and may not
reflect the rates observed in clinical practice.
In the rosuvastatin controlled clinical trials database (placebo or active-
controlled) of 5394 patients with a mean treatment duration of 15 weeks, 1.4%
of patients discontinued due to adverse reactions. The most common adverse
reactions that led to treatment discontinuation were:
• myalgia
• abdominal pain
• nausea
The most commonly reported adverse reactions (incidence ≥2%) in the
rosuvastatin controlled clinical trial database of 5394 patients were:
• headache
• myalgia
• abdominal pain
• asthenia
• nausea
Adverse reactions reported in ≥2% of patients in placebo-controlled clinical
studies and at a rate greater than placebo are shown in Table 1. These studies
had a treatment duration of up to 12 weeks.
Table 1. Adverse Reactions1Reported in ≥2% of Patients Treated with
Rosuvastatin and > Placebo in Placebo-Controlled Trials (% of Patients)
|
Adverse Reactions |
Rosuvastatin |
Rosuvastatin |
Rosuvastatin |
Rosuvastatin |
Total Rosuvastatin |
Placebo |
|
Headache |
5.5 |
4.9 |
3.1 |
8.5 |
5.5 |
5.0 |
|
Nausea |
3.8 |
3.5 |
6.3 |
0 |
3.4 |
3.1 |
|
Myalgia |
3.1 |
2.1 |
6.3 |
1.9 |
2.8 |
1.3 |
|
Asthenia |
2.4 |
3.2 |
4.7 |
0.9 |
2.7 |
2.6 |
|
Constipation |
2.1 |
2.1 |
4.7 |
2.8 |
2.4 |
2.4 |
1Adverse reactions by COSTART preferred term.
Other adverse reactions reported in clinical studies were abdominal pain,
dizziness, hypersensitivity (including rash, pruritus, urticaria, and
angioedema) and pancreatitis. The following laboratory abnormalities have also
been reported: dipstick-positive proteinuria and microscopic hematuria [see Warnings and Precautions (5.4)]; elevated creatine phosphokinase,
transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and
bilirubin; and thyroid function abnormalities.
In a clinical trial, involving 981 participants treated with rosuvastatin 40
mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years,
5.6% of subjects treated with rosuvastatin versus 2.8% of placebo-treated
subjects discontinued due to adverse reactions. The most common adverse
reactions that led to treatment discontinuation were: myalgia, hepatic enzyme
increased, headache, and nausea.
Adverse reactions reported in ≥2% of patients and at a rate greater than
placebo are shown in Table 2.
Table 2. Adverse Reactions1 Reported in ≥2% of Patients Treated with
Rosuvastatin and > Placebo in a Trial (% of Patients)
|
Adverse Reactions |
Rosuvastatin 40 mg |
Placebo |
|
Myalgia |
12.7 |
12.1 |
|
Arthralgia |
10.1 |
7.1 |
|
Headache |
6.4 |
5.3 |
|
Dizziness |
4.0 |
2.8 |
|
Increased CPK |
2.6 |
0.7 |
|
Abdominal pain |
2.4 |
1.8 |
|
ALT >3x ULN2 |
2.2 |
0.7 |
1 Adverse reactions by MedDRA preferred term.
2 Frequency recorded as abnormal laboratory value.
In a clinical trial, 17,802 participants were treated with rosuvastatin 20 mg
(n=8901) or placebo (n=8901) for a mean duration of 2 years. A higher
percentage of rosuvastatin-treated patients versus placebo-treated patients,
6.6% and 6.2%, respectively, discontinued study medication due to an adverse
event, irrespective of treatment causality. Myalgia was the most common
adverse reaction that led to treatment discontinuation.
There was a significantly higher frequency of diabetes mellitus reported in
patients taking rosuvastatin (2.8%) versus patients taking placebo (2.3%).
Mean HbA1c was significantly increased by 0.1% in rosuvastatin-treated
patients compared to placebo-treated patients. The number of patients with a
HbA1c >6.5% at the end of the trial was significantly higher in rosuvastatin-
treated versus placebo-treated patients [see Warnings and Precautions (5.5)].
Adverse reactions reported in ≥2% of patients and at a rate greater than
placebo are shown in Table 3.
Table 3. Adverse Reactions1 Reported in ≥2% of Patients Treated with
Rosuvastatin and > Placebo in a Trial (% of Patients)
|
Adverse Reactions |
Rosuvastatin 20 mg |
Placebo |
|
Myalgia |
7.6 |
6.6 |
|
Arthralgia |
3.8 |
3.2 |
|
Constipation |
3.3 |
3.0 |
|
Diabetes mellitus |
2.8 |
2.3 |
|
Nausea |
2.4 |
2.3 |
1 Treatment-emergent adverse reactions by MedDRA preferred term.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use
of rosuvastatin: arthralgia, fatal and non-fatal hepatic failure, hepatitis,
jaundice, thrombocytopenia, depression, sleep disorders (including insomnia
and nightmares), peripheral neuropathy, interstitial lung disease and
gynecomastia. Because these reactions are reported voluntarily from a
population of uncertain size, it is not always possible to reliably estimate
their frequency or establish a causal relationship to drug exposure.
There have been rare reports of immune-mediated necrotizing myopathy
associated with statin use [see Warnings and Precautions (5.1)].
There have been rare postmarketing reports of cognitive impairment (e.g.,
memory loss, forgetfulness, amnesia, memory impairment, and confusion)
associated with statin use. These cognitive issues have been reported for all
statins. The reports are generally nonserious, and reversible upon statin
discontinuation, with variable times to symptom onset (1 day to years) and
symptom resolution (median of 3 weeks).
Most frequent adverse reactions (rate ≥2%) are headache, myalgia, abdominal pain, asthenia, and nausea. (6.1)To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
The dose range for rosuvastatin tablets in adults is 5 to 40 mg orally once
daily. The usual starting dose is 10 to 20 mg once daily. The usual starting
dose in adult patients with homozygous familial hypercholesterolemia is 20 mg
once daily.
The maximum rosuvastatin tablets dose of 40 mg should be used only for those
patients who have not achieved their LDL-C goal utilizing the 20 mg dose [see Warnings and Precautions (5.1)].
Rosuvastatin tablets can be administered as a single dose at any time of day,
with or without food. The tablet should be swallowed whole.
When initiating rosuvastatin tablets therapy or switching from another HMG-CoA
reductase inhibitor therapy, the appropriate rosuvastatin tablets starting
dose should first be utilized, and only then titrated according to the
patient's response and individualized goal of therapy.
After initiation or upon titration of rosuvastatin tablets, lipid levels
should be analyzed within 2 to 4 weeks and the dosage adjusted accordingly.
Pediatric use information for patients 7 to 17 years of age is approved for
AstraZeneca's CRESTOR (rosuvastatin calcium) tablets. However, due to
AstraZeneca's marketing exclusivity rights, this drug product is not labeled
with that pediatric information.
2.3 Dosing in Asian Patients
In Asian patients, consider initiation of rosuvastatin tablets therapy with 5 mg once daily due to increased rosuvastatin plasma concentrations. The increased systemic exposure should be taken into consideration when treating Asian patients not adequately controlled at doses up to 20 mg/day [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
2.4 Use with Concomitant Therapy
Patients taking cyclosporine
The dose of rosuvastatin tablets should not exceed 5 mg once daily [see Warnings and Precautions (5.1), Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Patients taking gemfibrozil
Avoid concomitant use of rosuvastatin tablets with gemfibrozil. If concomitant
use cannot be avoided, initiate rosuvastatin tablets at 5 mg once daily. The
dose of rosuvastatin tablets should not exceed 10 mg once daily [see Warnings and Precautions (5.1), Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
Patients taking atazanavir and ritonavir, lopinavir and ritonavir, or
simeprevir
Initiate rosuvastatin tablets therapy with 5 mg once daily. The dose of
rosuvastatin tablets should not exceed 10 mg once daily [see Warnings and Precautions (5.1), Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
2.5 Dosing in Patients with Severe Renal Impairment
For patients with severe renal impairment (CLcr <30 mL/min/1.73 m2) not on hemodialysis, dosing of rosuvastatin tablets should be started at 5 mg once daily and not exceed 10 mg once daily [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
• Rosuvastatin tablets can be taken with or without food, at any time of day.
(2.1)
• Dose range: 5 to 40 mg once daily. Use 40 mg dose only for patients not
reaching LDL-C goal with 20 mg. (2.1)
• Adult HoFH: Starting dose 20 mg/day (2.1)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient
Information).
Patients should be instructed not to take 2 doses of rosuvastatin tablets
within 12 hours of each other.
Skeletal Muscle Effects
Patients should be advised to report promptly unexplained muscle pain,
tenderness, or weakness, particularly if accompanied by malaise or fever or if
these muscle signs or symptoms persist after discontinuing rosuvastatin
tablets.
Concomitant Use of Antacids
When taking rosuvastatin tablets with an aluminum and magnesium hydroxide
combination antacid, the antacid should be taken at least 2 hours after
rosuvastatin tablets administration.
Embryofetal Toxicity
Advise females of reproductive potential of the risk to a fetus, to use
effective contraception during treatment, and to inform their healthcare
provider of a known or suspected pregnancy. [see Contraindications(4) and Use in Specific Populations (8.1,8.3)].
Lactation
Advise women not to breastfeed during treatment with rosuvastatin tablets [see Contraindications (4) and Use in Specific Populations (8.2)].
Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation
of rosuvastatin tablets and if signs or symptoms of liver injury occur. All
patients treated with rosuvastatin tablets should be advised to promptly
report any symptoms that may indicate liver injury, including fatigue,
anorexia, right upper abdominal discomfort, dark urine or jaundice.

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:HETERO****TM
Hetero Labs Limited, Unit V, Polepally, Jadcherla,
Mahabubnagar - 509 301, India.
Revised: 10/2019
Repackaged By: Preferred Pharmaceuticals Inc.
