Montelukast
These highlights do not include all the information needed to use MONTELUKAST SODIUM TABLETS safely and effectively. See full prescribing information for MONTELUKAST SODIUM TABLETS. MONTELUKAST SODIUM tablets, for oral use Initial U.S. Approval: 1998
94642db1-5c75-44c4-92b4-8385b486df82
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
Preferred Pharmaceuticals, Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Montelukast
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
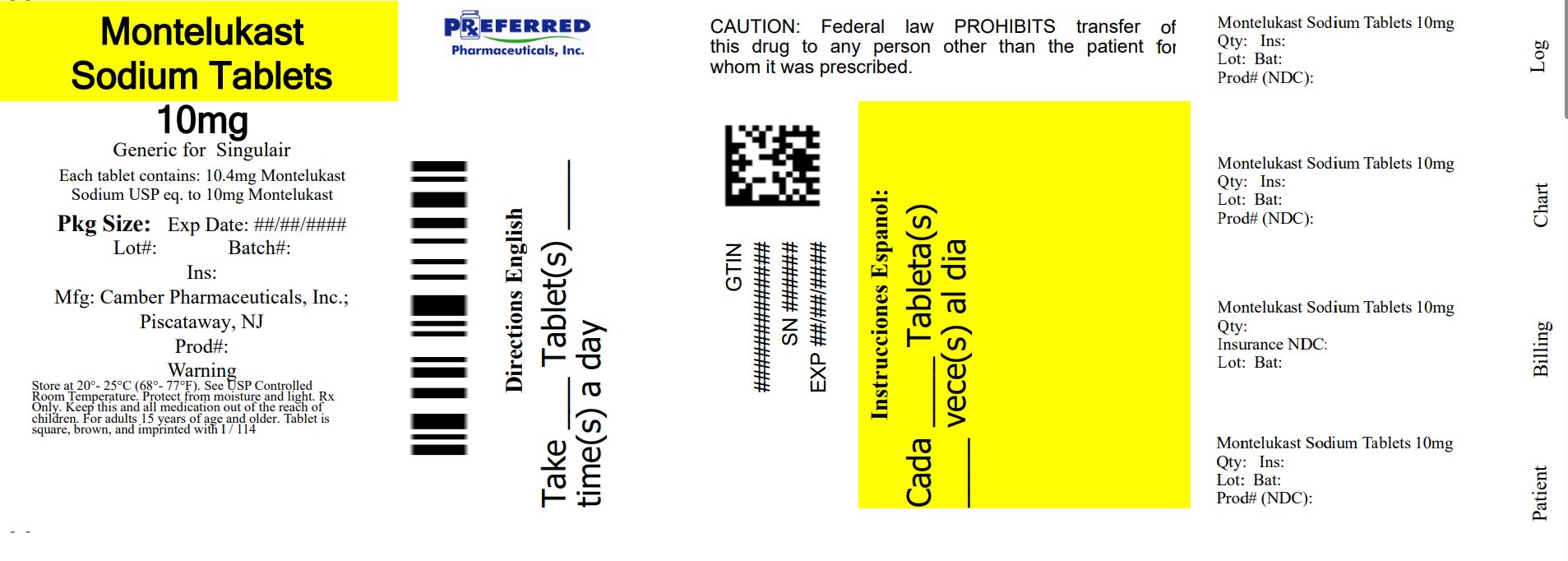
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Montelukast Sodium Tablets, 10 mg
Boxed Warning section
WARNING: SERIOUS NEUROPSYCHIATRIC EVENTS
See full prescribing information for complete boxed warning.
• Serious neuropsychiatric events have been reported in patients taking
montelukast sodium
• Discuss benefits and risks of montelukast sodium with patients and
caregivers
• Monitor for neuropsychiatric symptoms in patients taking montelukast sodium
• Discontinue montelukast sodium immediately if neuropsychiatric symptoms
occur
• Because the benefits of montelukast sodium may not outweigh the potential
risk of neuropsychiatric symptoms in patients with allergic rhinitis, reserve
use for patients who have an inadequate response or intolerance to alternative
therapies ( ,
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Asthma
Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 15 years of age and older.
1.2 Exercise-Induced Bronchoconstriction (EIB)
Montelukast sodium tablets are indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older.
1.3 Allergic Rhinitis
tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients of age and older. Because the benefits of montelukast sodium tablets may not outweigh the risk of neuropsychiatric symptoms in patients with reserve use for patients who have an inadequate response or intolerance to alternative therapies. Montelukast sodium tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients 15 years of age and older. Because the benefits of montelukast sodium tablets may not outweigh the risk of neuropsychiatric symptoms in patients with allergic rhinitis [see warnings and precautions reserve use for patients who have an inadequate response or intolerance to alternative therapies.
Montelukast sodium tablets are a leukotriene receptor antagonist indicated
for:
• Prophylaxis and chronic treatment of asthma in patients 15 years of age and
older
• Acute prevention of exercise-induced bronchoconstriction (EIB) in patients
15 years of age and older
• Relief of symptoms of allergic rhinitis (AR): seasonal allergic rhinitis
(SAR) in patients 15 years of age and older, and perennial allergic rhinitis
(PAR) in patients 15 years of age and older. Reserve use for patients who have
an inadequate response or intolerance to alternative therapies (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
• Hypersensitivity to any component of this product.
• Hypersensitivity to any component of this product (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Events
neuropsychiatric (NP) events have been reported with use of montelukast
sodium. These postmarketing reports have been highly variable and included,
but were not limited to, agitation, aggressive behavior or hostility,
anxiousness, depression, disorientation, disturbance in attention, dream
dysphemia (stuttering), hallucinations, insomnia, irritability, memory
impairment, obsessive-compulsive symptoms, restlessness, , suicidal thoughts
and behavior (including suicide), tic, and tremor. NP events have been
reported in adult, adolescent, and pediatric patients with and without a
history of psychiatric disorder. NP events have been reported mostly during
montelukast sodium treatment, but some were reported after sodium
discontinuation. Animal studies showed that montelukast distributes into the
brain in rats however, the mechanisms underlying montelukast sodium-associated
NP events are currently not well understood. Based upon the available data, it
is difficult to identify risk for or quantify the risk of NP events with
montelukast sodium use. of the risk of NP events, the benefits of montelukast
sodium may not outweigh the risks in some patients, particularly when the
symptoms of disease may be and adequately treated with alternative therapies.
Reserve use of montelukast sodium for patients with allergic rhinitis who have
an inadequate or intolerance to alternative therapies In patients with asthma
or exercise-induced bronchoconstriction, consider the benefits and before
prescribing montelukast sodium. the benefits and risks of montelukast sodium
use with patients and caregivers when prescribing montelukast sodium. Advise
patients and/or caregivers to be for changes in behavior or for new NP
symptoms when taking montelukast sodium. If changes in behavior are observed,
or if new NP symptoms or suicidal thoughts behavior occur, advise patients to
discontinue montelukast sodium and contact a healthcare provider immediately.
In many cases, resolved after stopping montelukast sodium therapy; however, in
some cases symptoms persisted after discontinuation of montelukast sodium.
continue to monitor and provide supportive care until symptoms resolve. Re-
evaluate the benefits and risks of restarting treatment with sodium if such
events occur. Serious neuropsychiatric (NP) events have been reported with use
of montelukast sodium. These postmarketing reports have been highly variable
and included, but were not limited to, agitation, aggressive behavior or
hostility, anxiousness, depression, disorientation, disturbance in attention,
dream abnormalities, dysphemia (stuttering), hallucinations, insomnia,
irritability, memory impairment, obsessive-compulsive symptoms, restlessness,
somnambulism, suicidal thoughts and behavior (including suicide), tic, and
tremor. NP events have been reported in adult, adolescent, and pediatric
patients with and without a previous history of psychiatric disorder. NP
events have been reported mostly during montelukast sodium treatment, but some
were reported after montelukast sodium discontinuation. Animal studies showed
that montelukast distributes into the brain in rats [see Clinical
Pharmacology however, the mechanisms underlying montelukast sodium-associated
NP events are currently not well understood. Based upon the available data, it
is difficult to identify risk factors for or quantify the risk of NP events
with montelukast sodium use.
Because of the risk of NP events, the benefits of montelukast sodium may not
outweigh the risks in some patients, particularly when the symptoms of disease
may be mild and adequately treated with alternative therapies. Reserve use of
montelukast sodium for patients with allergic rhinitis who have an inadequate
response or intolerance to alternative therapies [see Indications and Usage
In patients with asthma or exercise-induced bronchoconstriction, consider the
benefits and risks before prescribing montelukast sodium.
Discuss the benefits and risks of montelukast sodium use with patients and
caregivers when prescribing montelukast sodium. Advise patients and/or
caregivers to be alert for changes in behavior or for new NP symptoms when
taking montelukast sodium. If changes in behavior are observed, or if new NP
symptoms or suicidal thoughts and/or behavior occur, advise patients to
discontinue montelukast sodium and contact a healthcare provider immediately.
In many cases, symptoms resolved after stopping montelukast sodium therapy;
however, in some cases symptoms persisted after discontinuation of montelukast
sodium. Therefore, continue to monitor and provide supportive care until
symptoms resolve. Re-evaluate the benefits and risks of restarting treatment
with montelukast sodium if such events occur.
5.2 Acute Asthma
Montelukast sodium is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with montelukast sodium can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled β-agonist.
5.3 Concomitant Corticosteroid Use
While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast sodium should not be abruptly substituted for inhaled or oral corticosteroids.
5.4 Aspirin Sensitivity
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium. Although montelukast sodium is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti- inflammatory drugs in aspirin-sensitive asthmatic patients [see Clinical Studies
5.5 Eosinophilic Conditions
Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between montelukast sodium and these underlying conditions has not been established [see Adverse Reactions
• Do not prescribe montelukast sodium to treat an acute asthma attack
• Advise patients to have appropriate rescue medication available
• Inhaled corticosteroid may be reduced gradually. Do not abruptly substitute
montelukast sodium for inhaled or oral corticosteroids
• Patients with known aspirin sensitivity should continue to avoid aspirin or
non-steroidal anti-inflammatory agents while taking montelukast sodium
• Systemic eosinophilia, sometimes presenting with clinical features of
vasculitis consistent with Churg-Strauss syndrome, has been reported. These
events have been sometimes associated with the reduction of oral
corticosteroid therapy and (5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Adults and Adolescents 15 Years of Age and Older with Asthma
Montelukast sodium has been evaluated for safety in approximately 2950 adult
and adolescent patients 15 years of age and older in clinical trials. In
placebo-controlled clinical trials, the following adverse experiences reported
with montelukast sodium occurred in greater than or equal to 1% of patients
and at an incidence greater than that in patients treated with placebo:
Table 1: Adverse Experiences Occurring in ≥1% of Patients with an Incidence
Greater than that in Patients Treated with Placebo
|
Montelukast sodium |
Placebo | |
|
Body As A Whole | ||
|
Pain, abdominal |
2.9 |
2.5 |
|
Trauma |
1.0 2.1 |
0.8 |
|
1.1 | ||
|
Pain, dental |
1.7 |
1.0 |
|
Gastroenteritis, infectious |
1.5 |
0.5 |
|
Nervous System/Psychiatric | ||
|
Headache |
18.4 |
18.1 |
|
Dizziness |
1.9 |
1.4 |
|
Respiratory System Disorders | ||
|
Influenza |
4.2 |
3.9 |
|
Cough |
2.7 |
2.4 |
|
Congestion, nasal |
1.6 |
1.3 |
|
Skin/Skin Appendages Disorder | ||
|
Rash |
1.6 |
1.2 |
|
Laboratory Adverse Experiences* | ||
|
ALT increased |
2.1 |
2.0 |
|
AST increased |
1.6 |
1.2 |
|
Pyuria |
1.0 |
0.9 |
*Number of patients tested (montelukast sodium and placebo, respectively): ALT and AST, 1935, 1170; pyuria, 1924, 1159.
The frequency of less common adverse events was comparable between montelukast
sodium and placebo.
The safety profile of montelukast sodium, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for montelukast sodium.
Cumulatively, 569 patients were treated with montelukast sodium for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change.
Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. Montelukast sodium administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with montelukast sodium with a frequency ≥1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving montelukast sodium vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.
Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received montelukast sodium in two, 6-week, clinical studies. Montelukast sodium administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with montelukast sodium with a frequency ≥1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use
of montelukast sodium. Because these reactions are reported voluntarily from a
population of uncertain size, it is not always possible to reliably estimate
their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: increased bleeding tendency,
thrombocytopenia.
Immune system disorders: hypersensitivity reactions including anaphylaxis,
hepatic eosinophilic infiltration.
Psychiatric disorders: including, but not limited to, agitation, aggressive
behavior or hostility, anxiousness, depression, disorientation, disturbance in
attention, dream abnormalities, dysphemia (stuttering), hallucinations,
insomnia, irritability, memory impairment, obsessive-compulsive symptoms,
restlessness, somnambulism, suicidal thinking and behavior (including
suicide), tic, and tremor [see Boxed Warning, Warnings and Precautions
Nervous system disorders: drowsiness, paraesthesia/hypoesthesia, seizures.
Cardiac disorders: palpitations.
Respiratory, thoracic and mediastinal disorders: epistaxis, pulmonary
eosinophilia.
Gastrointestinal disorders: diarrhea, dyspepsia, nausea, pancreatitis,
vomiting.
Hepatobiliary disorders: Cases of cholestatic hepatitis, hepatocellular liver-
injury, and mixed-pattern liver injury have been reported in patients treated
with montelukast sodium. Most of these occurred in combination with other
confounding factors, such as use of other medications, or when montelukast
sodium was administered to patients who had underlying potential for liver
disease such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders: angioedema, bruising, erythema
multiforme, erythema nodosum, pruritus, Stevens-Johnson syndrome/toxic
epidermal necrolysis, urticaria.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia including
muscle cramps.
Renal and urinary disorders: enuresis in children.
General disorders and administration site conditions: edema.
Patients with asthma on therapy with montelukast sodium may present with
systemic eosinophilia, sometimes presenting with clinical features of
vasculitis consistent with Churg-Strauss syndrome, a condition which is often
treated with systemic corticosteroid therapy. These events have been sometimes
associated with the reduction of oral corticosteroid therapy. Physicians
should be alert to eosinophilia, vasculitic rash, worsening pulmonary
symptoms, cardiac complications, and/or neuropathy presenting in their
patients [see Warnings and Precautions
Most common adverse reactions (incidence ≥5% and greater than placebo listed in descending order of frequency): upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
No dose adjustment is needed when montelukast sodium is co-administered with theophylline, prednisone, prednisolone, oral contraceptives, terfenadine, digoxin, warfarin, gemfibrozil, itraconazole, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers [see Clinical Pharmacology
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of tumorigenicity was seen in carcinogenicity studies of either 2 years in Sprague-Dawley rats or 92 weeks in mice at oral gavage doses up to 200 mg/kg/day or 100 mg/kg/day, respectively. The estimated exposure in rats was approximately 120 and 75 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose. The estimated exposure in mice was approximately 45 and 25 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose.
Montelukast demonstrated no evidence of mutagenic or clastogenic activity in the following assays: the microbial mutagenesis assay, the V-79 mammalian cell mutagenesis assay, the alkaline elution assay in rat hepatocytes, the chromosomal aberration assay in Chinese hamster ovary cells, and in the in vivo mouse bone marrow chromosomal aberration assay.
In fertility studies in female rats, montelukast produced reductions in fertility and fecundity indices at an oral dose of 200 mg/kg (estimated exposure was approximately 70 times the AUC for adults at the maximum recommended daily oral dose). No effects on female fertility or fecundity were observed at an oral dose of 100 mg/kg (estimated exposure was approximately 20 times the AUC for adults at the maximum recommended daily oral dose). Montelukast had no effects on fertility in male rats at oral doses up to 800 mg/kg (estimated exposure was approximately 160 times the AUC for adults at the maximum recommended daily oral dose).
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The cysteinyl leukotrienes (LTC 4, LTD 4, LTE 4) are products of arachidonic
acid metabolism and are released from various cells, including mast cells and
eosinophils. These eicosanoids bind to cysteinyl leukotriene (CysLT)
receptors. The CysLT type-1 (CysLT 1) receptor is found in the human airway
(including airway smooth muscle cells and airway macrophages) and on other
pro-inflammatory cells (including eosinophils and certain myeloid stem cells).
CysLTs have been correlated with the pathophysiology of asthma and allergic
rhinitis. In asthma, leukotriene-mediated effects include airway edema, smooth
muscle contraction, and altered cellular activity associated with the
inflammatory process. In allergic rhinitis, CysLTs are released from the nasal
mucosa after allergen exposure during both early- and late-phase reactions and
are associated with symptoms of allergic rhinitis.
Montelukast is an orally active compound that binds with high affinity and
selectivity to the CysLT 1 receptor (in preference to other pharmacologically
important airway receptors, such as the prostanoid, cholinergic, or
β-adrenergic receptor). Montelukast inhibits physiologic actions of LTD 4 at
the CysLT 1 receptor without any agonist activity.
12.2 Pharmacodynamics
Montelukast causes inhibition of airway cysteinyl leukotriene receptors as demonstrated by the ability to inhibit bronchoconstriction due to inhaled LTD 4 in asthmatics. Doses as low as 5 mg cause substantial blockage of LTD 4-induced bronchoconstriction. In a placebo-controlled, crossover study (n=12), montelukast sodium inhibited early- and late-phase bronchoconstriction due to antigen challenge by 75% and 57%, respectively.
The effect of montelukast sodium on eosinophils in the peripheral blood was examined in clinical trials. In patients with asthma aged 2 years and older who received montelukast sodium, a decrease in mean peripheral blood eosinophil counts ranging from 9% to 15% was noted, compared with placebo, over the double-blind treatment periods. In patients with seasonal allergic rhinitis aged 15 years and older who received montelukast sodium, a mean increase of 0.2% in peripheral blood eosinophil counts was noted, compared with a mean increase of 12.5% in placebo-treated patients, over the double- blind treatment periods; this reflects a mean difference of 12.3% in favor of montelukast sodium. The relationship between these observations and the clinical benefits of montelukast noted in the clinical trials is not known [see Clinical Studies (14)].
12.3 Pharmacokinetics
Absorption
Montelukast is rapidly absorbed following oral administration. After
administration of the 10 mg film-coated tablet to fasted adults, the mean peak
montelukast plasma concentration (C max) is achieved in 3 to 4 hours (T max).
The mean oral bioavailability is 64%. The oral bioavailability and C max are
not influenced by a standard meal in the morning.
The safety and efficacy of montelukast sodium in patients with asthma were
demonstrated in clinical trials in which the 10 mg film-coated tablet
formulation was administered in the evening without regard to the time of food
ingestion. The safety and efficacy of montelukast sodium in patients with
seasonal allergic rhinitis were demonstrated in clinical trials in which the
10 mg film-coated tablet was administered in the morning or evening without
regard to the time of food ingestion.
The comparative pharmacokinetics of montelukast when administered as two 5 mg
chewable tablets versus one 10 mg film-coated tablet have not been evaluated.
Distribution
Montelukast is more than 99% bound to plasma proteins. The steady state volume
of distribution of montelukast averages 8 to 11 liters. Orally administered
montelukast distributes into the brain in rats.
Metabolism
Montelukast is extensively metabolized. In studies with therapeutic doses,
plasma concentrations of metabolites of montelukast are undetectable at steady
state in adults and pediatric patients.
In vitro studies using human liver microsomes indicate that CYP3A4, 2C8, and
2C9 are involved in the metabolism of montelukast. At clinically relevant
concentrations, 2C8 appears to play a major role in the metabolism of
montelukast.
Elimination
The plasma clearance of montelukast averages 45 mL/min in healthy adults.
Following an oral dose of radiolabeled montelukast, 86% of the radioactivity
was recovered in 5-day fecal collections and <0.2% was recovered in urine.
Coupled with estimates of montelukast oral bioavailability, this indicates
that montelukast and its metabolites are excreted almost exclusively via the
bile.
In several studies, the mean plasma half-life of montelukast ranged from 2.7
to 5.5 hours in healthy young adults. The pharmacokinetics of montelukast are
nearly linear for oral doses up to 50 mg. During once-daily dosing with 10 mg
montelukast, there is little accumulation of the parent drug in plasma (14%).
Special Populations
Hepatic Insufficiency: Patients with mild-to-moderate hepatic insufficiency
and clinical evidence of cirrhosis had evidence of decreased metabolism of
montelukast resulting in 41% (90% CI=7%, 85%) higher mean montelukast AUC
following a single 10 mg dose. The elimination of montelukast was slightly
prolonged compared with that in healthy subjects (mean half-life, 7.4 hours).
No dosage adjustment is required in patients with mild-to-moderate hepatic
insufficiency. The pharmacokinetics of montelukast sodium in patients with
more severe hepatic impairment or with hepatitis have not been evaluated.
Renal Insufficiency: Since montelukast and its metabolites are not excreted in
the urine, the pharmacokinetics of montelukast was not evaluated in patients
with renal insuffciency. No dosage adjustment is recommended in these
patients.
Gender: The pharmacokinetics of montelukast is similar in males and females.
Race: Pharmacokinetic differences due to race have not been studied.
Adolescents and Pediatric Patients: Pharmacokinetic studies evaluated the
systemic exposure of the 10 mg film-coated tablets in young adults and
adolescents ≥15 years of age.
The plasma concentration profile of montelukast following administration of
the 10 mg film-coated tablet is similar in adolescents ≥15 years of age and
young adults. The 10 mg film-coated tablet is recommended for use in patients
≥15 years of age.
Drug-Drug Interactions
Theophylline, Prednisone, and Prednisolone: Montelukast sodium has been
administered with other therapies routinely used in the prophylaxis and
chronic treatment of asthma with no apparent increase in adverse reactions. In
drug-interaction studies, the recommended clinical dose of montelukast did not
have clinically important effects on the pharmacokinetics of the following
drugs: theophylline, prednisone, and prednisolone.
Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady
state, did not cause clinically significant changes in the kinetics of a
single intravenous dose of theophylline [predominantly a cytochrome P450 (CYP) 1A2 substrate]. Montelukast at doses of ≥100 mg daily dosed to pharmacokinetic
steady state, did not cause any clinically significant change in plasma
profiles of prednisone or prednisolone following administration of either oral
prednisone or intravenous prednisolone.
Oral Contraceptives, Terfenadine, Digoxin, and Warfarin: In drug interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: oral contraceptives (norethindrone 1 mg/ ethinyl estradiol 35 mcg), terfenadine, digoxin, and warfarin. Montelukast at doses of ≥100 mg daily dosed to pharmacokinetic steady state did not significantly alter the plasma concentrations of either component of an oral contraceptive containing norethindrone 1 mg/ethinyl estradiol 35 mcg. Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady state did not change the plasma concentration profile of terfenadine (a substrate of CYP3A4) or fexofenadine, the carboxylated metabolite, and did not prolong the QTc interval following co-administration with terfenadine 60 mg twice daily; did not change the pharmacokinetic profile or urinary excretion of immunoreactive digoxin; did not change the pharmacokinetic profile of warfarin (primarily a substrate of CYP2C9, 3A4 and 1A2) or influence the effect of a single 30 mg oral dose of warfarin on prothrombin time or the International Normalized Ratio (INR).
Thyroid Hormones, Sedative Hypnotics, Non-Steroidal Anti-Inflammatory Agents,
Benzodiazepines, and Decongestants: Although additional specific interaction
studies were not performed, montelukast sodium was used concomitantly with a
wide range of commonly prescribed drugs in clinical studies without evidence
of clinical adverse interactions. These medications included thyroid hormones,
sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines,
and decongestants.
Cytochrome P450 (CYP) Enzyme Inducers: Phenobarbital, which induces hepatic
metabolism, decreased the area under the plasma concentration curve (AUC) of
montelukast approximately 40% following a single 10 mg dose of montelukast. No
dosage adjustment for montelukast sodium is recommended. It is reasonable to
employ appropriate clinical monitoring when potent CYP enzyme inducers, such
as phenobarbital or rifampin, are co-administered with montelukast sodium.
Effect of Montelukast on Cytochrome P450 (CYP) Enzymes: Montelukast is a
potent inhibitor of CYP2C8 in vitro. However, data from a clinical drug-drug
interaction study involving montelukast and rosiglitazone (a probe substrate
representative of drugs primarily metabolized by CYP2C8) in 12 healthy
individuals demonstrated that the pharmacokinetics of rosiglitazone are not
altered when the drugs are coadministered, indicating that montelukast does
not inhibit CYP2C8 in vivo. Therefore, montelukast is not anticipated to alter
the metabolism of drugs metabolized by this enzyme (e.g., paclitaxel,
rosiglitazone, and repaglinide). Based on further in vitro results in human
liver microsomes, therapeutic plasma concentrations of montelukast do not
inhibit CYP 3A4, 2C9, 1A2, 2A6, 2C19, or 2D6.
Cytochrome P450 (CYP) Enzyme Inhibitors: In vitro studies have shown that
montelukast is a substrate of CYP 2C8, 2C9, and 3A4. Co-administration of
montelukast with itraconazole, a strong CYP 3A4 inhibitor, resulted in no
significant increase in the systemic exposure of montelukast. Data from a
clinical drug-drug interaction study involving montelukast and gemfibrozil (an
inhibitor of both CYP 2C8 and 2C9) demonstrated that gemfibrozil, at a
therapeutic dose, increased the systemic exposure of montelukast by 4.4-fold.
Co-administration of itraconazole, gemfibrozil, and montelukast did not
further increase the systemic exposure of montelukast. Based on available
clinical experience, no dosage adjustment of montelukast is required upon co-
administration with gemfibrozil [see Overdosage
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Asthma
Adults and Adolescents 15 Years of Age and Older with Asthma
Clinical trials in adults and adolescents 15 years of age and older
demonstrated there is no additional clinical benefit to montelukast doses
above 10 mg once daily.
The efficacy of montelukast sodium for the chronic treatment of asthma in
adults and adolescents 15 years of age and older was demonstrated in two (U.S.
and Multinational) similarly designed, randomized, 12-week, double-blind,
placebo-controlled trials in 1576 patients (795 treated with montelukast
sodium, 530 treated with placebo, and 251 treated with active control). The
median age was 33 years (range 15 to 85); 56.8% were females and 43.2% were
males. The ethnic/racial distribution in these studies was 71.6% Caucasian,
17.7% Hispanic, 7.2% other origins and 3.5% Black. Patients had mild or
moderate asthma and were non-smokers who required approximately 5 puffs of
inhaled β-agonist per day on an "as-needed" basis. The patients had a mean
baseline percent of predicted forced expiratory volume in 1 second (FEV 1) of
66% (approximate range, 40 to 90%). The co-primary endpoints in these trials
were FEV 1 and daytime asthma symptoms. In both studies after 12 weeks, a
random subset of patients receiving montelukast sodium was switched to placebo
for an additional 3 weeks of double-blind treatment to evaluate for possible
rebound effects.
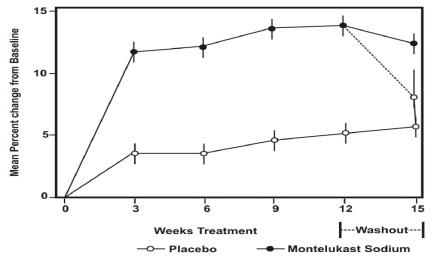
The results of the U.S. trial on the primary endpoint, morning FEV 1, expressed as mean percent change from baseline averaged over the 12-week treatment period, are shown in FIGURE 2. Compared with placebo, treatment with one montelukast sodium 10 mg tablet daily in the evening resulted in a statistically significant increase in FEV 1 percent change from baseline (13.0%-change in the group treated with montelukast sodium vs. 4.2%-change in the placebo group, p<0.001); the change from baseline in FEV 1 for montelukast sodium was 0.32 liters compared with 0.10 liters for placebo, corresponding to a between-group difference of 0.22 liters (p<0.001, 95% CI 0.17 liters, 0.27 liters). The results of the Multinational trial on FEV 1 were similar.
** Figure 2: FEV1 Mean Percent Change from Baseline**
(U.S. Trial: Montelukast sodium N=406; Placebo N=270)
(ANOVA Model)
****
The effect of montelukast sodium on other primary and secondary endpoints, represented by the Multinational study is shown in TABLE 2. Results on these endpoints were similar in the US study.
Table 2: Effect of Montelukast Sodium on Primary and Secondary Endpoints in a Multinational Placebo-controlled Trial (ANOVA Model)
|
Montelukast sodium |
Placebo | |||||
|
Endpoint |
N |
Baseline |
Mean Change from Baseline |
N |
Baseline |
Mean Change from Baseline |
|
Daytime Asthma Symptoms |
372 |
2.35 |
-0.49 * |
245 |
2.40 |
-0.26 |
|
β-agonist (puffs per day) |
371 |
5.35 |
-1.65 * |
241 |
5.78 |
-0.42 |
|
AM PEFR (L/min) |
372 |
339.57 |
25.03 * |
244 |
335.24 |
1.83 |
|
PM PEFR (L/min) |
372 |
355.23 |
20.13 * |
244 |
354.02 |
-0.49 |
|
Nocturnal Awakenings (#/week) |
285 |
5.46 |
-2.03 * |
195 |
5.57 |
-0.78 |
- p<0.001, compared with placebo
Both studies evaluated the effect of montelukast sodium on secondary outcomes, including asthma attack (utilization of health-care resources such as an unscheduled visit to a doctor's office, emergency room, or hospital; or treatment with oral, intravenous, or intramuscular corticosteroid), and use of oral corticosteroids for asthma rescue. In the Multinational study, significantly fewer patients (15.6% of patients) on montelukast sodium experienced asthma attacks compared with patients on placebo (27.3%, p<0.001). In the US study, 7.8% of patients on montelukast sodium and 10.3% of patients on placebo experienced asthma attacks, but the difference between the two treatment groups was not significant (p=0.334). In the Multinational study, significantly fewer patients (14.8% of patients) on montelukast sodium were prescribed oral corticosteroids for asthma rescue compared with patients on placebo (25.7%, p<0.001). In the US study, 6.9% of patients on montelukast sodium and 9.9% of patients on placebo were prescribed oral corticosteroids for asthma rescue, but the difference between the two treatment groups was not significant (p=0.196).
Onset of Action and Maintenance of Effects
In each placebo-controlled trial in adults, the treatment effect of montelukast sodium, measured by daily diary card parameters, including symptom scores, "as-needed" β-agonist use, and PEFR measurements, was achieved after the first dose and was maintained throughout the dosing interval (24 hours). No significant change in treatment effect was observed during continuous once- daily evening administration in non-placebo-controlled extension trials for up to one year. Withdrawal of montelukast sodium in asthmatic patients after 12 weeks of continuous use did not cause rebound worsening of asthma.
Effects in Patients on Concomitant Inhaled Corticosteroids
Separate trials in adults evaluated the ability of montelukast sodium to add to the clinical effect of inhaled corticosteroids and to allow inhaled corticosteroid tapering when used concomitantly.
One randomized, placebo-controlled, parallel-group trial (n=226) enrolled adults with stable asthma with a mean FEV 1 of approximately 84% of predicted who were previously maintained on various inhaled corticosteroids (delivered by metered-dose aerosol or dry powder inhalers). The median age was 41.5 years (range 16 to 70); 52.2% were females and 47.8% were males. The ethnic/racial distribution in this study was 92.0% Caucasian, 3.5% Black, 2.2% Hispanic, and 2.2% Asian. The types of inhaled corticosteroids and their mean baseline requirements included beclomethasone dipropionate (mean dose, 1203 mcg/day), triamcinolone acetonide (mean dose, 2004 mcg/day), flunisolide (mean dose, 1971 mcg/day), fluticasone propionate (mean dose, 1083 mcg/day), or budesonide (mean dose, 1192 mcg/day). Some of these inhaled corticosteroids were non-U.S.-approved formulations, and doses expressed may not be ex-actuator. The pre-study inhaled corticosteroid requirements were reduced by approximately 37% during a 5- to 7-week placebo run-in period designed to titrate patients toward their lowest effective inhaled corticosteroid dose. Treatment with montelukast sodium resulted in a further 47% reduction in mean inhaled corticosteroid dose compared with a mean reduction of 30% in the placebo group over the 12-week active treatment period (p≤0.05). It is not known whether the results of this study can be generalized to patients with asthma who require higher doses of inhaled corticosteroids or systemic corticosteroids.
In another randomized, placebo-controlled, parallel-group trial (n=642) in a similar population of adult patients previously maintained, but not adequately controlled, on inhaled corticosteroids (beclomethasone 336 mcg/day), the addition of montelukast sodium to beclomethasone resulted in statistically significant improvements in FEV 1 compared with those patients who were continued on beclomethasone alone or those patients who were withdrawn from beclomethasone and treated with montelukast or placebo alone over the last 10 weeks of the 16-week, blinded treatment period. Patients who were randomized to treatment arms containing beclomethasone had statistically significantly better asthma control than those patients randomized to montelukast sodium alone or placebo alone as indicated by FEV 1, daytime asthma symptoms, PEFR, nocturnal awakenings due to asthma, and "as-needed" β-agonist requirements.
In adult patients with asthma with documented aspirin sensitivity, nearly all of whom were receiving concomitant inhaled and/or oral corticosteroids, a 4-week, randomized, parallel-group trial (n=80) demonstrated that montelukast sodium, compared with placebo, resulted in significant improvement in parameters of asthma control. The magnitude of effect of montelukast sodium in aspirin-sensitive patients was similar to the effect observed in the general population of asthma patients studied. The effect of montelukast sodium on the bronchoconstrictor response to aspirin or other non-steroidal anti- inflammatory drugs in aspirin-sensitive asthmatic patients has not been evaluated [see Warnings and Precautions
14.2 Exercise-Induced Bronchoconstriction (EIB)
Exercise-Induced Bronchoconstriction (Adults and Adolescents 15 years of age and older)
The efficacy of montelukast sodium, 10 mg, when given as a single dose 2 hours before exercise for the prevention of EIB was investigated in three (U.S. and Multinational), randomized, double-blind, placebo-controlled crossover studies that included a total of 160 adult and adolescent patients 15 years of age and older with EIB. Exercise challenge testing was conducted at 2 hours, 8.5 or 12 hours, and 24 hours following administration of a single dose of study drug (montelukast sodium 10 mg or placebo). The primary endpoint was the mean maximum percent fall in FEV 1 following the 2 hours post-dose exercise challenge in all three studies (Study A, Study B, and Study C). In Study A, a single dose of montelukast sodium 10 mg demonstrated a statistically significant protective benefit against EIB when taken 2 hours prior to exercise. Some patients were protected from EIB at 8.5 and 24 hours after administration; however, some patients were not. The results for the mean maximum percent fall at each timepoint in Study A are shown in TABLE 3 and are representative of the results from the other two studies.
** Table 3 : Mean Maximum Percent Fall in FEV1 Following Exercise Challenge in Study A (N=47) ANOVA Model**
|
Time of exercise challenge following medication administration |
Mean Maximum percent fall in FEV 1* |
Treatment | |
|
Montelukast sodium |
Placebo | ||
|
2 hours |
13 |
22 |
-9 (-12, -5) |
|
8.5 hours |
12 |
17 |
-5 (-9, -2) |
|
24 hours |
10 |
14 |
-4 (-7, -1) |
*Least squares-mean
Daily administration of montelukast sodium for the chronic treatment of asthma
has not been established to prevent acute episodes of EIB.
In a 12-week, randomized, double-blind, parallel group study of 110 adult and
adolescent asthmatics 15 years of age and older, with a mean baseline FEV 1
percent of predicted of 83% and with documented exercise-induced exacerbation
of asthma, treatment with montelukast sodium, 10 mg, once daily in the
evening, resulted in a statistically significant reduction in mean maximal
percent fall in FEV 1 and mean time to recovery to within 5% of the pre-
exercise FEV 1. Exercise challenge was conducted at the end of the dosing
interval (i.e., 20 to 24 hours after the preceding dose). This effect was
maintained throughout the 12-week treatment period indicating that tolerance
did not occur. Montelukast sodium did not, however, prevent clinically
significant deterioration in maximal percent fall in FEV 1 after exercise
(i.e., ≥20% decrease from pre-exercise baseline) in 52% of patients studied.
In a separate crossover study in adults, a similar effect was observed after
two once-daily 10 mg doses of montelukast sodium.
14.3 Allergic Rhinitis (Seasonal and Perennial)
Seasonal Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of seasonal allergic rhinitis was investigated in 5 similarly designed, randomized, double-blind, parallel-group, placebo- and active-controlled (loratadine) trials conducted in North America. The 5 trials enrolled a total of 5029 patients, of whom 1799 were treated with montelukast sodium tablets. Patients were 15 to 82 years of age with a history of seasonal allergic rhinitis, a positive skin test to at least one relevant seasonal allergen, and active symptoms of seasonal allergic rhinitis at study entry.
The period of randomized treatment was 2 weeks in 4 trials and 4 weeks in one trial. The primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing) as assessed by patients on a 0 to 3 categorical scale.
Four of the five trials showed a significant reduction in daytime nasal symptoms scores with montelukast sodium 10 mg tablets compared with placebo. The results of one trial are shown below. The median age in this trial was 35.0 years (range 15 to 81); 65.4% were females and 34.6% were males. The ethnic/racial distribution in this study was 83.1% Caucasian, 6.4% other origins, 5.8% Black, and 4.8% Hispanic. The mean changes from baseline in daytime nasal symptoms score in the treatment groups that received montelukast sodium tablets, loratadine, and placebo are shown in TABLE 5. The remaining three trials that demonstrated efficacy showed similar results.
Table 5: Effects of Montelukast Sodium on Daytime Nasal Symptoms Score*
in a Placebo- and Active-controlled Trial in Patients with Seasonal Allergic Rhinitis
(ANCOVA Model)
|
Treatment Group (N) |
Baseline Mean Score |
Mean Change from Baseline |
Difference Between Treatment and Placebo (95% CI) |
|
Montelukast sodium |
2.09 |
-0.39 |
-0.13 † (-0.21, -0.06) |
|
Placebo (351) |
2.10 |
-0.26 |
N.A. |
|
Active Control ‡ |
2.06 |
-0.46 |
-0.24 † (-0.31, -0.17) |
- Average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing as assessed by
patients on a 0 to 3 categorical scale.
† Statistically different from placebo (p≤0.001).
‡ The study was not designed for statistical comparison between montelukast sodium and the active control (loratadine).
Perennial Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of perennial allergic rhinitis was investigated in 2 randomized, double-blind, placebo- controlled studies conducted in North America and Europe. The two studies enrolled a total of 3357 patients, of whom 1632 received montelukast sodium 10 mg tablets. Patients 15 to 82 years of age with perennial allergic rhinitis as confirmed by history and a positive skin test to at least one relevant perennial allergen (dust mites, animal dander, and/or mold spores), who had active symptoms at the time of study entry, were enrolled.
In the study in which efficacy was demonstrated, the median age was 35 years (range 15 to 81); 64.1% were females and 35.9% were males. The ethnic/racial distribution in this study was 83.2% Caucasian, 8.1% Black, 5.4% Hispanic, 2.3% Asian, and 1.0% other origins. Montelukast sodium 10 mg tablets once daily was shown to significantly reduce symptoms of perennial allergic rhinitis over a 6-week treatment period (TABLE 6); in this study the primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, and sneezing).
Table 6: Effects of Montelukast Sodium on Daytime Nasal Symptoms Score in a Placebo-controlled Trial in Patients with Perennial Allergic Rhinitis (ANCOVA Model)*
|
Treatment Group (N) |
Baseline |
Mean Change from Baseline |
Difference Between Treatment and Placebo (95% CI) |
|
Montelukast sodium |
2.09 |
-0.42 |
- 0.08 † (-0.12, -0.04) |
|
Placebo |
2.10 |
-0.35 |
N.A. |
*Average of individual scores of nasal congestion, rhinorrhea, sneezing as assessed by patients on a 0 to 3 categorical scale.
†Statistically different from placebo (p≤0.001).
The other 6-week study evaluated montelukast sodium 10 mg (n=626), placebo (n=609), and an active-control (cetirizine 10 mg; n=120). The primary analysis compared the mean change from baseline in daytime nasal symptoms score for montelukast sodium vs. placebo over the first 4 weeks of treatment; the study was not designed for statistical comparison between montelukast sodium and the active-control. The primary outcome variable included nasal itching in addition to nasal congestion, rhinorrhea, and sneezing. The estimated difference between montelukast sodium and placebo was -0.04 with a 95% CI of (-0.09, 0.01). The estimated difference between the active-control and placebo was -0.10 with a 95% CI of (-0.19, -0.01).
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Montelukast Sodium Tablets, USP
** (mon" te loo' kast soe' dee um)**
What is the most important information I should know about montelukast sodium tablets?
Serious mental health problems have happened in people taking montelukast sodium tablets or even after treatment has stopped. This can happen in people with or without a history of mental health problems. Stop taking montelukast sodium tablets and tell your healthcare provider right away if you or your child have any unusual changes in behavior or thinking, including any of these symptoms:
• agitation, including aggressive behavior or hostility
• attention problems
• bad or vivid dreams
• depression
• disorientation (confusion)
• feeling anxious
• irritability
• hallucinations (seeing or hearing things that are not really there)
• memory problems
• obsessive-compulsive symptoms
• restlessness
• sleep walking
• stuttering
• suicidal thoughts and actions (including suicide)
• tremor
• trouble sleeping
• uncontrolled muscle movements
What are montelukast sodium tablets?
Montelukast sodium tablets are a prescription medicine that blocks substances in the body called leukotrienes. This may help to improve symptoms of asthma and inflammation of the lining of the nose (allergic rhinitis). Montelukast sodium tablets do not contain a steroid.
Montelukast sodium tablets are used to:
1. Prevent asthma attacks and for the long-term treatment of asthma in adults and adolescents ages 15 years and older.
Do not take montelukast sodium tablets if you need relief right away for a sudden asthma attack. If you have an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
2. Prevent exercise-induced asthma in people 15 years of age and older.
3. Help control the symptoms of allergic rhinitis such as sneezing, stuffy
nose, runny nose, and itching of the nose. Montelukast sodium tablets are used
to treat the following in people who have already taken other medicines that
did not work well enough or in people who could not tolerate other medicines:
• outdoor allergies that happen part of the year (seasonal allergic rhinitis)
in adults and adolescents ages 15 years and older,** and**
• indoor allergies that happen all year (perennial allergic rhinitis) in
adults and adolescents ages 15 years and older.
Do not take montelukast sodium tablets if you are allergic to any of its ingredients. See the end of this Medication Guide for a complete list of the ingredients in montelukast sodium tablets.
Before taking montelukast sodium tablets, tell your healthcare provider about all your medical conditions, including if you:
• are allergic to aspirin
• have or have had mental health problems.
• are pregnant or plan to become pregnant. Talk to your healthcare provider if
you are pregnant or plan to become pregnant, montelukast sodium tablets may
not be right for you.
• are breastfeeding or plan to breastfeed. It is not known if montelukast
sodium passes into your breast milk. Talk to your healthcare provider about
the best way to feed your baby while taking montelukast sodium tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over the counter medicines, vitamins, and herbal supplements. Some medicines may affect how montelukast sodium tablets work, or montelukast sodium tablets may affect how your other medicines work.
How should I take montelukast sodium tablets?
Foranyone who takes montelukast sodium tablets:
• Take montelukast sodium tablets exactly as prescribed by your healthcare
provider. Your healthcare provider will tell you how much montelukast sodium
tablets to take, andwhen to take it.
• Stop taking montelukast sodium tablets and tell your healthcare provider
right away if you or your child have any unusual changes in behavior or
thinking.
• You can take montelukast sodium tablets with food or without food.
•** If you or your child misses a dose of montelukast sodium tablets, just
take the next dose at your regular time.** Do not take 2 doses at the same
time.
• If you take too much montelukast sodium, call your healthcare provider right
away.
For adults and adolescents 15 years of age and older with asthma:
• Take montelukast sodium tablets 1 time each day, in the evening. Continue to
take montelukast sodium tablets every day for as long as your healthcare
provider prescribes it, even if you have no asthma symptoms.
• Tell your healthcare provider right away if your asthma symptoms get worse,
or if you need to use your rescue inhaler medicine more often for asthma
attacks.
• Always have your rescue inhaler medicine with you for asthma attacks.
• Continue to take your other asthma medicines as prescribed unless your
healthcare provider tells you to change how you take these medicines.
For people 15 years of age and older for the prevention of exercise-induced
asthma:
• Take montelukast sodium tablets at least 2 hours before exercise.
• Always have your rescue inhaler medicine with you for asthma attacks.
• If you take montelukast sodium tablets every day for chronic asthma or
allergic rhinitis,** do not** take another dose to prevent exercise-induced
asthma. Talk to your healthcare provider about your treatment for exercise-
induced asthma.
• Do not take 2 doses of montelukast sodium tablets within 24 hours (1
day).
For anyone 15 years of age and older with seasonal allergic rhinitis, or for
anyone 15 years of age and older with perennial allergic rhinitis:
• Take montelukast sodium tablets 1 time each day, at about the same time each
day.
What should I avoid while taking montelukast sodium tablets?
If you have asthma and aspirin makes your asthma symptoms worse, continue to
avoid taking aspirin or other medicines called non-steroidal anti-inflammatory
drugs (NSAIDs) while taking montelukast sodium tablets.
What are the possible side effects of montelukast sodium tablets?
Montelukast sodium tablets may cause serious side effects, including:
** •See "What is the most important information I should know about
montelukast sodium tablets?"**
** • Increase in certain white blood cells (eosinophils) and possible inflamed
blood vessels throughout the body (systemic vasculitis).** Rarely, this can
happen in people with asthma who take montelukast sodium tablets. This
sometimes happens in people who also take a steroid medicine by mouth that is
being stopped or the dose is being lowered.
Tell your healthcare provider right away if you get one or more of these
symptoms:
o a feeling of pins and needles or numbness of arms or legs
o a flu-like illness
o rash
o severe inflammation (pain and swelling) of the sinuses (sinusitis)
The most common side effects of montelukast sodium tablets include:
• upper respiratory infection
• fever
• headache
• sore throat
• cough
• stomach pain
• diarrhea
• earache or ear infection
• flu
• runny nose
• sinus infection
These are not all the possible side effects of montelukast sodium tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store montelukast sodium tablets?
• Store montelukast sodium tablets at room temperature between 68° to 77°F
(20° to 25° C).
• Keep montelukast sodium tablets in the package they come in.
• Keep montelukast sodium tablets in a dry place and keep it away from light.
• Keep montelukast sodium tablets and all medicines out of reach of
children.
General information about the safe and effective use of montelukast sodium
tablets
Medicines are sometimes prescribed for purposes other than those listed in a
Medication Guide. Do not use montelukast sodium tablets for a condition for
which it was not prescribed. Do not give montelukast sodium tablets to other
people even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about
montelukast sodium tablets that is written for health professionals.
For more information, call Hetero Labs Limited at 1-866-495-1995.
What are the ingredients in montelukast sodium tablets?
Active ingredient: montelukast sodium USP
Inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, mannitol and microcrystalline cellulose. The tablets are coated with opadry yellow which contains carnauba wax, hydroxypropyl cellulose, hypromellose, red iron oxide, titanium dioxide and yellow iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration
Medication Guide available at http://camberpharma.com/medication-guides

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:HETERO****TM
Hetero Labs Limited, Unit V, Polepally,
Jadcherla, Mahabubnagar – 509 301, India.
or
By:
HETERO****TM
Hetero Labs Limited
Jeedimetla, Hyderabad-500 055, India.
Revised: 08/2020
Repackaged By: Preferred Pharmaceuticals, Inc.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Boxed Warning 04/2020
Indications and Usage, Allergic Rhinitis 04/2020
Dosage and Administration, Asthma
Allergic Rhinitis Asthma and Allergic Rhinitis 04/2020
Warnings and Precautions, Neuropsychiatric Events 04/2020
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Asthma
Montelukast sodium tablets should be taken once daily in the evening. The
following doses are recommended: For adults and adolescents 15 years of age
and older: one 10 mg tablet. Safety and effectiveness in pediatric patients
less than 12 months of age with asthma have not been established. who miss a
dose should take the next dose at their regular time and should not take 2
doses at the same time. There have been no clinical trials in patients with
asthma to evaluate the relative efficacy of morning versus evening dosing. The
pharmacokinetics of montelukast are similar whether dosed in the morning or
evening. Efficacy has been demonstrated for asthma when montelukast was
administered in the evening without regard to time of food ingestion.
Montelukast sodium tablets should be taken once daily in the evening. The
following doses are recommended:
For adults and adolescents 15 years of age and older: one 10 mg tablet.
Safety and effectiveness in pediatric patients less than 12 months of age with
asthma have not been established.
Patients who miss a dose should take the next dose at their regular time and
should not take 2 doses at the same time.
There have been no clinical trials in patients with asthma to evaluate the
relative efficacy of morning versus evening dosing. The pharmacokinetics of
montelukast are similar whether dosed in the morning or evening. Efficacy has
been demonstrated for asthma when montelukast was administered in the evening
without regard to time of food ingestion.
2.2 Exercise-Induced Bronchoconstriction (EIB)
For prevention of EIB, a single dose of montelukast sodium tablets should be
taken at least 2 hours before exercise.
The following doses are recommended:
For adults and adolescents 15 years of age and older: one 10 mg tablet.
An additional dose of montelukast sodium tablets should not be taken within 24
hours of a previous dose. Patients already taking montelukast sodium tablets
daily for another indication (including chronic asthma) should not take an
additional dose to prevent EIB. All patients should have available for rescue
a short-acting β-agonist. Safety and efficacy in patients younger than 6 years
of age have not been established. Daily administration of montelukast sodium
tablets for the chronic treatment of asthma has not been established to
prevent acute episodes of EIB.
2.3 Allergic Rhinitis
For allergic rhinitis, montelukast sodium tablets should be taken once daily.
Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was
administered in the morning or the evening without regard to time of food
ingestion. The time of administration may be individualized to suit patient
needs. The following doses for the treatment of symptoms of seasonal allergic
rhinitis are recommended: For adults and adolescents 15 years of age and
older: one 10 mg tablet. Safety and effectiveness in pediatric patients
younger than 2 years of age with seasonal allergic rhinitis have not been
established. The following doses for the treatment of symptoms of perennial
allergic rhinitis are recommended: For adults and adolescents 15 years of age
and older: one 10 mg tablet. Safety and effectiveness in pediatric patients
younger than 6 months of age with perennial allergic rhinitis have not been
established. who miss a dose should take the next dose at their regular time
and should not take 2 doses at the same time. For allergic rhinitis,
montelukast sodium tablets should be taken once daily. Efficacy was
demonstrated for seasonal allergic rhinitis when montelukast was administered
in the morning or the evening without regard to time of food ingestion. The
time of administration may be individualized to suit patient needs.
The following doses for the treatment of symptoms of seasonal allergic
rhinitis are recommended:
For adults and adolescents 15 years of age and older: one 10 mg tablet.
Safety and effectiveness in pediatric patients younger than 2 years of age
with seasonal allergic rhinitis have not been established.
The following doses for the treatment of symptoms of perennial allergic
rhinitis are recommended:
For adults and adolescents 15 years of age and older: one 10 mg tablet.
Safety and effectiveness in pediatric patients younger than 6 months of age
with perennial allergic rhinitis have not been established.
Patients who miss a dose should take the next dose at their regular time and
should not take 2 doses at the same time.
2.4 Asthma and Allergic Rhinitis
Patients with both asthma and allergic rhinitis should take only one
montelukast sodium tablets dose daily in the evening. who miss a dose should
take the next dose at their regular time and should not take 2 doses at the
same time. Patients with both asthma and allergic rhinitis should take only
one montelukast sodium tablets dose daily in the evening.
Patients who miss a dose should take the next dose at their regular time and
should not take 2 doses at the same time.
Administration (by indications):
• Asthma Once daily in the evening for patients 15 years and older.
• Acute prevention of EIB One tablet at least 2 hours before exercise for
patients 15 years of age and older.
• Seasonal allergic rhinitis Once daily for patients 15 years and older.
• Perennial allergic rhinitis Once daily for patients 15 years and older.
Dosage (by age) (2):
• 15 years and older: one 10 mg tablet.
Patients with both asthma and allergic rhinitis should take only one dose daily in the evening
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published prospective and retrospective cohort studies
over decades with montelukast use in pregnant women have not established a
drug-associated risk of major birth defects [see Data]. In animal reproduction
studies, no adverse developmental effects were observed with oral
administration of montelukast to pregnant rats and rabbits during
organogenesis at doses approximately 100 and 110 times, respectively, the
maximum recommended human daily oral dose (MRHDOD) based on AUCs [see Data].
The estimated background risk of major birth defects and miscarriage for the
indicated population is unknown. All pregnancies have a background risk of
birth defect, loss, or other adverse outcomes. In the U.S. general population,
the estimated background risk of major birth defects and miscarriage in
clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly or moderately controlled asthma in pregnancy increases the maternal
risk of perinatal adverse outcomes such as preeclampsia and infant
prematurity, low birth weight, and small for gestational age.
Data
Human Data
Published data from prospective and retrospective cohort studies have not
identified an association with montelukast sodium use during pregnancy and
major birth defects. Available studies have methodologic limitations,
including small sample size, in some cases retrospective data collection, and
inconsistent comparator groups.
Animal Data
In embryo-fetal development studies, montelukast administered to pregnant rats
and rabbits during organogenesis (gestation days 6 to 17 in rats and 6 to 18
in rabbits) did not cause any adverse developmental effects at maternal oral
doses up to 400 and 300 mg/kg/day in rats and rabbits, respectively
(approximately 100 and 110 times the AUC in humans at the MRHDOD,
respectively).
8.2 Lactation
Risk Summary
A published clinical lactation study reports the presence of montelukast in human milk. Data available on the effects of the drug on infants, either directly [see Use in Specific Populations or through breast milk, do not suggest a significant risk of adverse events from exposure to montelukast sodium. The effects of the drug on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for montelukast sodium and any potential adverse effects on the breastfed infant from montelukast sodium or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients below the age of 12 months with asthma, 6 months with perennial allergic rhinitis, and 6 years with exercise-induced bronchoconstriction have not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10 mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required.
8.6 Hepatic Insufficiency
No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency [see Clinical Pharmacology (
8.7 Renal Insufficiency
No dosage adjustment is recommended in patients with renal insufficiency [see Clinical Pharmacology
DESCRIPTION SECTION
11 DESCRIPTION
Montelukast sodium USP, the active ingredient in montelukast sodium tablets, USP is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT 1 receptor.
Montelukast sodium USP is described chemically as [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl] cyclopropaneacetic acid, monosodium salt.
The empirical formula is C 35H 35CINNaO 3S, and its molecular weight is 608.17. The structural formula is:
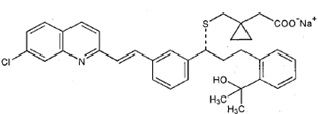
Montelukast sodium USP is a hygroscopic, optically active, white or almost white powder. Montelukast sodium USP is freely soluble in water and methylene chloride, freely soluble to very soluble in alcohol.
Each 10 mg film-coated montelukast sodium tablet USP contains 10.4 mg montelukast sodium USP, which is equivalent to 10 mg of montelukast, and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, mannitol and microcrystalline cellulose. The tablets are coated with opadry yellow which contains carnauba wax, hydroxypropyl cellulose, hypromellose, red iron oxide, titanium dioxide and yellow iron oxide.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
For the tablets, advise the patient and/or caregiver to read the FDA-approved
patient labeling (Medication Guide).
• Advise patients about the potential risk for serious neuropsychiatric
symptoms and behavioral changes with montelukast sodium use.
• Discuss the benefits and risks of montelukast sodium with patients when
prescribing or continuing treatment with montelukast sodium.
• Advise patients to monitor for changes in behavior or neuropsychiatric
symptoms in patients taking montelukast sodium.
• Instruct patients to discontinue montelukast sodium and contact a healthcare
provider immediately if changes in behavior or thinking that are not typical
for the patient occur, or if the patient develops suicidal ideation or
suicidal behavior.
• Advise patients to take montelukast sodium daily as prescribed, even when
they are asymptomatic, as well as during periods of worsening asthma, and to
contact their physicians if their asthma is not well controlled.
• Advise Patients that oral montelukast sodium is not for the treatment of
acute asthma attacks. They should have appropriate short-acting inhaled
β-agonist medication available to treat asthma exacerbations. Patients who
have exacerbations of asthma after exercise should be instructed to have
available for rescue a short-acting inhaled β-agonist. Daily administration of
montelukast sodium for the chronic treatment of asthma has not been
established to prevent acute episodes of EIB.
• Advise patients to seek medical attention if short-acting inhaled
bronchodilators are needed more often than usual, or if more than the maximum
number of inhalations of short-acting bronchodilator treatment prescribed for
a 24-hour period are needed.
• Instruct patients to continue other anti-asthma medications as prescribed
unless instructed by a physician.
• Instruct patients with known aspirin sensitivity to continue avoidance of
aspirin or non-steroidal anti-inflammatory agents while taking montelukast
sodium.

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:HETEROTM
Hetero Labs Limited, Unit V, Polepally,
Jadcherla, Mahabubnagar – 509 301, India.
or
By:
HETEROTM
Hetero Labs Limited
Jeedimetla, Hyderabad-500 055, India.
Revised: 08/2020
Repackaged By: Preferred Pharmaceuticals, Inc.
