Allergy Relief
CVS Pharmacy, Inc. Allergy Relief Drug Facts
7cac266a-4de8-4af1-8324-b01823a3989a
HUMAN OTC DRUG LABEL
Apr 16, 2025
CVS Pharmacy
DUNS: 062312574
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fexofenadine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
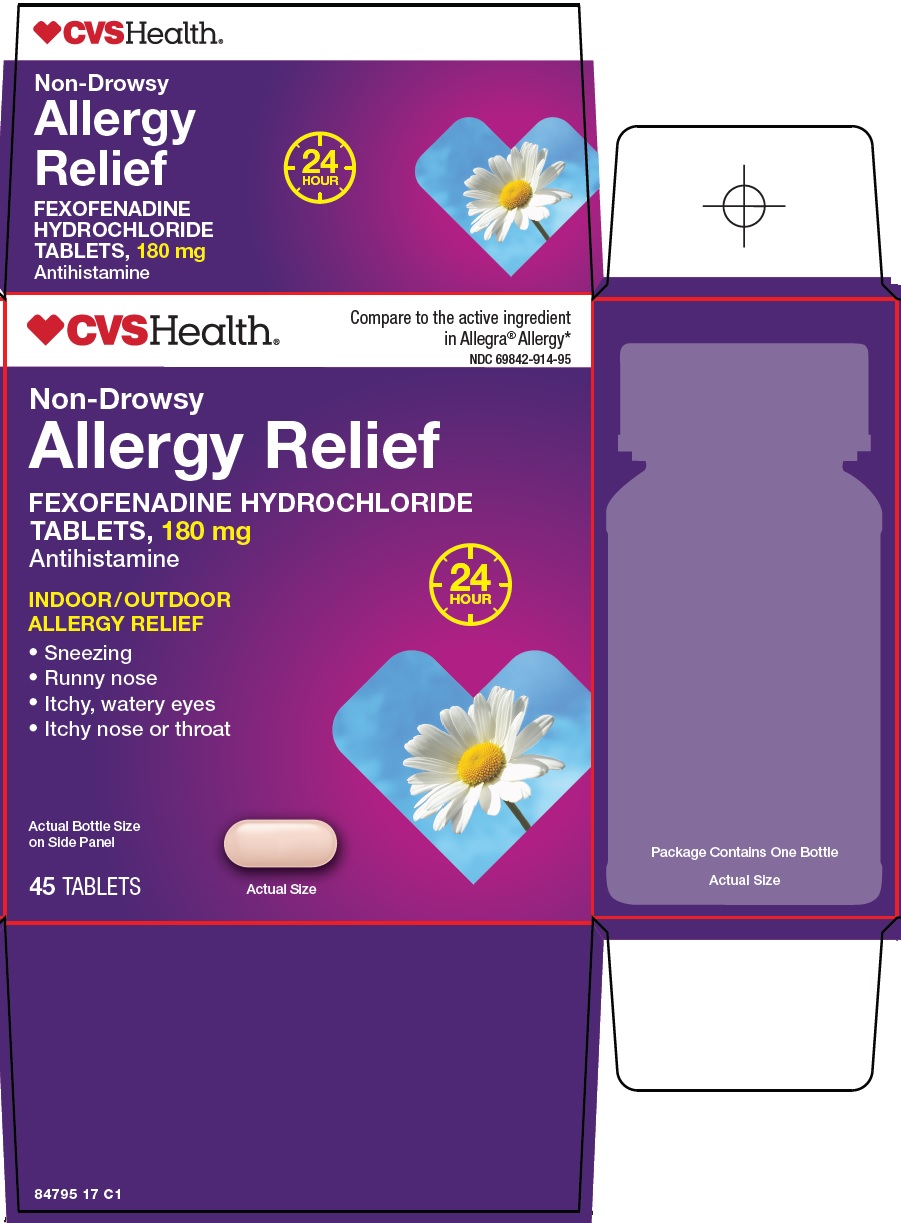
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
CVS Health®
Compare to the active ingredient in Allegra® Allergy
Non-Drowsy
Allergy Relief
FEXOFENADINE HYDROCHLORIDE TABLETS, 180 mg
Antihistamine
INDOOR/OUTDOOR ALLERGY RELIEF
24 HOUR
● Sneezing
● Runny nose
● Itchy, watery eyes
● Itchy nose or throat
Actual Bottle Size on Side Panel
45 TABLETS
Actual Size

STORAGE AND HANDLING SECTION
Other information
•
do not use if printed foil under cap is broken or missing
•
store between 20° -25°C (68° -77°F)
•
protect from excessive moisture
•
this product meets the requirements of USP Dissolution Test 2
OTC - QUESTIONS SECTION
Questions or comments?
1-800-719-9260
