Verkazia
These highlights do not include all the information needed to use VERKAZIA safely and effectively. See full prescribing information for VERKAZIA. Verkazia® (cyclosporine ophthalmic emulsion) 0.1%, for topical ophthalmic use Initial U.S. Approval: 1983
c795cd2f-89da-78e3-e053-2a95a90a9422
HUMAN PRESCRIPTION DRUG LABEL
Oct 12, 2023
Santen Incorporated
DUNS: 869321331
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
cyclosporine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Eye Injury and Contamination
To avoid the potential for eye injury or contamination, advise patients not to touch the vial tip to the eye or other surfaces.
To avoid the potential for eye injury and contamination, advise patient not to touch the vial tip to the eye or other surfaces ( 5.1)
DESCRIPTION SECTION
11 DESCRIPTION
Verkazia (cyclosporine ophthalmic emulsion) 0.1% contains a topical calcineurin inhibitor immunosuppressant. Cyclosporine is a white or almost white powder. Cyclosporine’s chemical name is Cyclo[[( E)-(2 S,3 R,4 R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl- N-methylglycyl- N-methyl-L-leucyl-L-valyl- N-methyl-L-leucyl-L-alanyl-D- alanyl- N-methyl-L-leucyl- N-methyl-L-leucyl- N-methyl-L-valyl] and it has the following structure:
Structural Formula
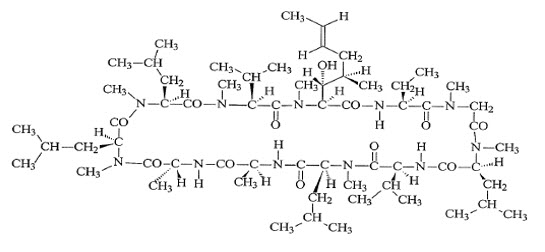
Formula: C 62H 111N 11O 12 Mol. Wt.: 1202.61
Verkazia ophthalmic emulsion is a sterile, unpreserved topical emulsion. It appears as a milky-white homogeneous emulsion. It has an osmolality of approximately 265 mOsmol/kg and a pH of 5-7. Each mL of Verkazia ophthalmic emulsion contains: Active: Cyclosporine 1 mg/mL. Inactives: Cetalkonium chloride, Glycerol, Medium-chain triglycerides, Poloxamer 188, Sodium Hydroxide to adjust pH, Tyloxapol and Water for Injection.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Systemic carcinogenicity studies were carried out in male and female mice and rats. In the 78-week oral (diet) mouse study, at doses of 1, 4, and 16 mg/kg/day, evidence of a statistically significant trend was found for lymphocytic lymphomas in females, and the incidence of hepatocellular carcinomas in mid-dose males significantly exceeded the control value. The low dose in mice is approximately 5 times greater than MRHOD.
In the 24-month oral (diet) rat study, conducted at 0.5, 2, and 8 mg/kg/day, pancreatic islet cell adenomas significantly exceeded the control rate in the low dose level. The hepatocellular carcinomas and pancreatic islet cell adenomas were not dose related. The low dose in rats is approximately 5 times greater than MRHOD.
Mutagenesis
In genetic toxicity tests, cyclosporine has not been found to be mutagenic/genotoxic in the Ames Test, the V79-HGPRT Test, the micronucleus test in mice and Chinese hamsters, the chromosome-aberration tests in Chinese hamster bone-marrow, the mouse dominant lethal assay, and the DNA-repair test in sperm from treated mice. Cyclosporine was positive in an in vitro sister chromatid exchange (SCE) assay using human lymphocytes.
Impairment of Fertility
Oral administration of cyclosporine to rats for 12 weeks (male) and 2 weeks
(female) prior to mating produced no adverse effects on fertility at doses up
to 15 mg/kg/day
(160 times higher than MRHOD).
PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Handling the Vial
Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may contaminate the emulsion. Advise patients to not touch the vial tip to their eye to avoid the potential for injury to the eye [see Warnings and Precautions ( 5.1)].
Use with Contact Lenses
Advise patients that contact lenses should be removed before administering Verkazia and to wait at least 15 minutes after instillation of the dose before reinserting the contact lenses [see Dosage and Administration ( 2.1)] .
Administration
Advise patients that the emulsion from one individual single-dose vial is to be used immediately after opening for administration to one or both eyes, and the remaining contents should be discarded immediately after administration [see Dosage and Administration ( 2) ].
Missed Dose
If a dose is missed, Verkazia should be continued as normal at the next dose as planned. [see Dosage and Administration ( 2.1)] .
Manufactured for: Santen Inc.
Manufactured by: ExcelVision
Distributed by:
Santen Inc.
6401 Hollis Street, Suite 125
Emeryville, CA 94608
United States
