LODOCO
ff06d68f-d65f-d097-e053-6294a90a7e5f
HUMAN PRESCRIPTION DRUG LABEL
Aug 24, 2023
AGEPHA Pharma USA, LLC
DUNS: 117633746
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Colchicine Tablets 0.5 mg
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
ADVERSE REACTIONS SECTION
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
In the LoDoCo2 trial, myalgia was reported for 21.2% of individuals randomized to colchicine and 18.5% of individuals randomized to matching placebo (hazard ratio 1.15, 95% CI 1.01-1.31).
6.2 Postmarketing Experience
The following adverse reactions have been identified with colchicine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
These most often occur with excessive accumulation or overdosage [see Overdosage (10)].
Neuromuscular: myotoxicity, weakness, numbness, paresthesia, rhabdomyolysis.
Gastrointestinal: diarrhea, vomiting, abdominal cramping, abdominal pain.
Renal: acute renal impairment
Dermatology: rashes and alopecia
Hematological: thrombocytopenia, leukopenia, pancytopenia
DRUG INTERACTIONS SECTION
7. DRUG INTERACTIONS
Colchicine is a substrate for the efflux transporter P-glycoprotein (P-gp). CYP3A4 is the primary enzyme involved in the metabolism of colchicine. If LODOCO is administered with drugs that inhibit P-gp, most of which also inhibit CYP3A4, increased concentrations of colchicine are likely (Table 1).
Table 1: Drug Interactions
|
Drug class |
Outcome/effect |
Clinical comment |
|
Strong CYP3A4 Inhibitors† atazanavir |
Significant increases in colchicine plasma levels [see Clinical Pharmacology (12.3)]. |
Concomitant use of LODOCO with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4)]. |
|
Moderate CYP3A4 amprenavir |
Significant increase in colchicine plasma concentration is anticipated. |
Monitor patients receiving moderate CYP3A4 inhibitors for |
|
Grapefruit grapefruit juice |
Grapefruit juice increases exposure to colchicine. |
Advise patients to avoid grapefruit or grapefruit juice when taking LODOCO. |
|
P- glycoprotein Inhibitors† cyclosporine |
Significant increase in colchicine plasma levels is anticipated with P-gp inhibitors. |
Concomitant use of LODOCO with strong P-gp inhibitors is |
|
HMG-Co A Reductase |
Pharmacokinetic and/or pharmacodynamic interaction: the addition of one drug to a stable long term regimen of the other has resulted in myopathy and rhabdomyolysis (including fatality). |
Monitor patients for signs or symptoms of muscle pain toxicity. |
|
Other Lipid Lowering drugs fibrates | ||
|
Digitalis Glycosides digoxin |
P-gp substrate; rhabdomyolysis has been reported. | |
|
Oral contraceptives Ethinyl estradiol and |
In healthy female volunteers given coadministered with 0.6 mg colchicine twice daily, hormone concentrations are not affected [see Clinical Pharmacology (12.3)]. Colchicine can interact with oral contraceptives like norethindrone/ethinyl estradiol and can cause adverse events like diarrhea, nausea, upper abdominal pain, cold sweat etc. |
Females using oral contraceptives |
† Patients with renal or hepatic impairment should not be given LODOCO in conjunction with strong CYP3A4 or P-gp inhibitors [see Contraindications (4)].
USE IN SPECIFIC POPULATIONS SECTION
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
Available human data from published literature on colchicine use in pregnancy over several decades have not identified any drug associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Colchicine crosses the human placenta. Although animal reproduction and developmental studies were not conducted with LODOCO (colchicine), published animal reproduction and development studies indicate that colchicine causes embryofetal toxicity and altered postnatal development at exposures within or above the clinical therapeutic range.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects or miscarriage in pregnant women with rheumatic diseases (such as rheumatoid arthritis, Behcet’s disease, or Familial Mediterranean fever (FMF)) treated with colchicine at therapeutic doses during pregnancy.
Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
8.2 Lactation
Risk summary
Colchicine is present in human milk (see Data). Adverse events in breastfed infants have not been reported in the published literature after administration of colchicine to lactating women. There are no data on the effects of colchicine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LODOCO and any potential adverse effects on the breastfed infant from LODOCO or from the underlying maternal condition.
Data
Limited published data from case reports and a small lactation study
demonstrate colchicine is present in breastmilk. A systematic review of
literature reported no adverse effects in 149 breastfed children and advised
to reconsider breastfeeding if the infant has diarrhea. In a prospective
observational cohort study, no gastrointestinal or other symptoms were
reported in 38 colchicineexposed
breastfed infants.
8.3 Females and Males of Reproductive Potential
Infertility
Case reports and epidemiology studies in human male subjects on colchicine
therapy indicated that infertility from colchicine is rare and may be
reversible.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
8.5 Geriatric Use
The total number of subjects included in the LoDoCo2 study who were 65 and
over and 75 and over were 3098 (56.1%). No overall differences in safety or
effectiveness were observed between these subjects and younger subjects, and
other reported clinical experience has not identified differences in responses
between the elderly and younger patients, but greater sensitivity of some
older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
8.6 Renal Impairment
Colchicine is known to be excreted renally. In renally impaired patients, the total clearance of colchicine is significantly reduced. In patients, with renal impairment, elevated plasma concentrations of colchicine were observed, and may lead to the development of colchicine toxicity and adverse reactions like myeloneuropathy characterized by proximal weakness and elevated serum creatinine, and possibly rhabdomyolysis [see Warnings and Precautions (5)].
LODOCO is contraindicated in patients with renal failure (Creatinine clearance less than 15 mL/minute). Patients with renal impairment should be monitored closely for adverse effects of colchicine [see Contraindications (4)].
Avoid use in patients with moderate renal impairment receiving moderate CYP3A4 inhibitors [see Contraindications (4), Warnings and Precautions (5) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Colchicine is primarily metabolized in liver. The clearance of colchicine may be significantly reduced, and plasma half-life prolonged in patients with chronic hepatic impairment, compared to healthy subjects [see Clinical Pharmacology (12.3)]. In patients with hepatic impairment, monitor closely for adverse reactions with LODOCO. LODOCO use in severe hepatic impairment is contraindicated. Avoid use in patients with any degree of hepatic impairment and receiving strong P-gp inhibitors or strong or moderate CYP3A4 inhibitors [see Contraindications (4) and Clinical Pharmacology (12.3)].
CLINICAL STUDIES SECTION
14. CLINICAL STUDIES
The evidence for the efficacy of colchicine in patients with cardiovascular
events is derived from published literature (LoDoCo2) along with other
supportive studies.
The LoDoCo2 trial was an investigator-initiated, randomized, placebo
controlled, double-blind, event-driven trial conducted to assess the efficacy
of colchicine 0.5mg once daily orally in patients with stable coronary artery
disease. The study included 5522 patients, in which 2762 were assigned to the
colchicine group and 2760 to placebo. The baseline characteristics of the
patients were balanced between the trial groups. Patients were treated for
chronic coronary disease, with 99.7% taking an antiplatelet agent or an
anticoagulant, 96.6% a lipid-lowering agent, 97.3% of which were statins,
62.1% a beta-blocker, and 71.7% an inhibitor of the renin–angiotensin system.
LoDoCo2 assessed the efficacy of colchicine 0.5 mg once daily in patients with
stable coronary artery disease. Patients between 35-85 years were eligible for
participation in the LoDoCo2 trial if they had proven coronary artery disease,
were clinically stable for at least 6 months, without comorbidities that would
preclude follow-up, or contraindication to colchicine use. The mean (±SD) age
of the patients was 66±8.6 years, and 846 (15.3%) were female. The median time
on study medication was 28.6 months. The primary end point of LoDoCo2 trial
was a composite of cardiovascular death, spontaneous (nonprocedural)
myocardial infarction, ischemic stroke, or ischemia-driven coronary
revascularization.
In the LoDoCo2 trial, 0.5 mg of colchicine once daily resulted in a 31% lower relative risk (RRR) of the primary composite endpoint events compared to placebo (HR, 0.69; 95% [CI] 0.57 to 0.83; p<0.001) and the number needed to treat (NNT) was 36.
Figure 1: Cumulative Incidence of Primary End point in LoDoCo2 trial
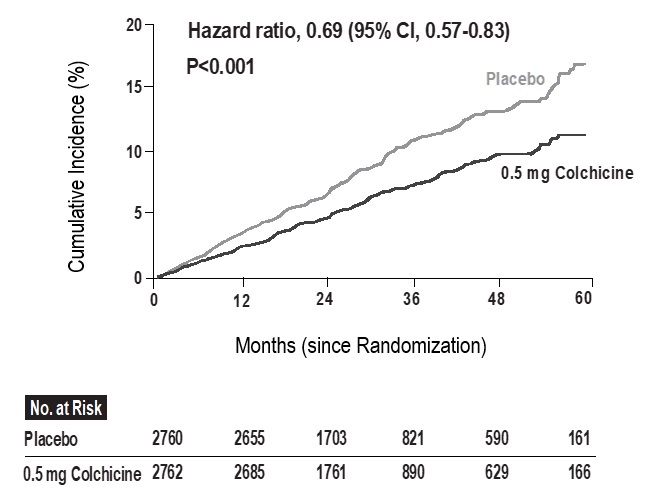
The results of the primary endpoint are summarized in Table 4.
Table 4: Summary of efficacy results of LoDoCo2 trial
|
LoDoCo2 Trial | ||||
|
End Point |
Colchicine |
Placebo |
Hazard |
P value |
|
Primary end point |
187 (6.8) |
264 (9.6) |
0.69 |
<0.001 |
|
Ischemia-driven coronary revascularization |
135 (4.9) |
177 (6.4) |
0.75 | |
|
Myocardial infarction |
83 (3.0) |
116 (4.2) |
0.70 | |
|
Ischemic stroke |
16 (0.6) |
24 (0.9) |
0.66 | |
|
Cardiovascular death |
20 (0.7) |
25 (0.9) |
0.80 |
Myocardial infarction refers to spontaneous (non-procedural) myocardial infarction.
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
|
PATIENT INFORMATION LODOCO (Low-Doe-Co) (colchicine) tablets, for oral use |
|
What is LODOCO? LODOCO is a prescription medicine used to reduce the risk of heart attack, stroke, certain types of heart procedures, and cardiovascular death in adults with: known buildup of plaque inside the arteries (atherosclerotic disease),or with multiple risk factors for cardiovascular disease. |
|
Do not take LODOCO if you: take certain medicines called strong CYP3A4 inhibitors or P-glycoprotein
inhibitors. Ask your healthcare provider if you are not sure. Taking certain
medicines with LODOCO may cause your level of LODOCO to be too high in your
body and cause life-threatening side effects or death. |
|
Before taking LODOCO, tell your healthcare provider about all your medical conditions, including if you: have liver or kidney problems. are breastfeeding or plan to breastfeed. LODOCO can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with LODOCO. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take: certain medicines called moderate CYP3A4 inhibitors antibiotics Ask your healthcare provider or pharmacist if you are not sure if you take any of the medicines listed above. This is not a complete list of all the medicines that can interact with LODOCO. Taking LODOCO with certain other medicines can affect each other and cause
serious side effects or death. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take LODOCO? Take LODOCO exactly as your healthcare provider tells you to take it. If you take too much LODOCO, go to the nearest hospital emergency room right away. |
|
What should I avoid while taking LODOCO? Avoid eating grapefruit or drinking grapefruit juice while taking LODOCO. Grapefruit and grapefruit juice may increase your chance of side effects while taking LODOCO. |
|
What are the possible side effects of LODOCO? LODOCO may cause serious side effects, including: Blood problems. LODOCO can cause low red blood cell counts, low white blood cell counts, and low platelet counts, which can be life-threatening or may lead to death. Muscle weakness (neuromuscular toxicity). LODOCO can cause muscle weakness and muscle problems. Stop taking LODOCO and get medical help right away if you get muscle pain or weakness, or tingling or numbness in your fingers or toes. |
|
The most common side effects of LODOCO include: diarrhea, vomiting, and stomach-area (abdominal) cramping. Tell your
healthcare provider right away if you develop any of these symptoms, they are
often the first sign of having too much LODOCO in your body. LODOCO may cause temporary fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if this is a concern for you. Tell your healthcare provider if you have any side effect that is new, that bothers you or that does not go away. These are not all of the possible side effects of LODOCO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to AGEPHA Pharma FZ LLC at 1-800-963-0353. |
|
How should I store LODOCO? Store LODOCO at room temperature between 68°F to 77°F (20°C to 25°C). Keep LODOCO and all medicines out of the reach of children. |
|
General information about the safe and effective use of LODOCO. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LODOCO for a condition for which it was not prescribed. Do not give LODOCO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LODOCO that is written for health professionals. |
|
What are the ingredients in LODOCO? Active Ingredient: colchicine. Inactive Ingredients: gelatin, lactose monohydrate, magnesium stearate, potato starch, and talc. Distributed by: AGEPHA Pharma USA, LLC, 181 New Road, Suite 304, Parsippany, NJ 07054 For more information, go to www.LODOCO.com or call 1-800-963-0353. |
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 06/2023
