Lamotrigine
These highlights do not include all the information needed to use LAMOTRIGINE TABLETS and LAMOTRIGINE TABLETS FOR ORAL SUSPENSION safely and effectively. See full prescribing information for LAMOTRIGINE TABLETS and LAMOTRIGINE TABLETS FOR ORAL SUSPENSION. LAMOTRIGINE tablets, for oral use LAMOTRIGINE tablets for oral suspension Initial U.S. Approval: 1994
e1fc15ef-b4aa-4dfa-bb12-cc706be2e12d
HUMAN PRESCRIPTION DRUG LABEL
Jul 7, 2025
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lamotrigine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Lamotrigine
GENERIC: Lamotrigine
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 70518-2473-0
NDC: 70518-2473-1
NDC: 70518-2473-2
COLOR: white
SHAPE: ROUND
SCORE: Two even pieces
SIZE: 13 mm
IMPRINT: ZC;82
PACKAGING: 1 in 1 POUCH
OUTER PACKAGING: 100 in 1 BOX
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- LAMOTRIGINE 200mg in 1
INACTIVE INGREDIENT(S):
- LACTOSE MONOHYDRATE
- MAGNESIUM STEARATE
- SODIUM STARCH GLYCOLATE TYPE A POTATO
- POVIDONE
- CELLULOSE, MICROCRYSTALLINE

DESCRIPTION SECTION
11 DESCRIPTION
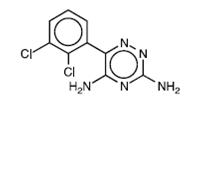
Lamotrigine, an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Lamotrigine's chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)- as-triazine, its molecular formula is C 9H 7N 5Cl 2, and its molecular weight is 256.09. Lamotrigine, USP is a white to pale cream-colored powder and has a pK aof 5.7. Lamotrigine is very slightly soluble in water (0.17 mg/mL at 25°C) and slightly soluble in 0.1 M HCl (4.1 mg/mL at 25°C). The structural formula is:
Each lamotrigine tablet, USP intended for oral administration contains 25 mg or 50 mg or 100 mg or 150 mg or 200 mg or 250 mg of lamotrigine. In addition, each tablet contains the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium starch glycolate.
Each lamotrigine tablets for oral suspension, USP intended for oral administration contains 5 mg or 25 mg of lamotrigine. In addition, each tablet contains the following inactive ingredients: aspartame, croscarmellose sodium, flavour black currant, magnesium stearate, mannitol, microcrystalline cellulose, silicon dioxide and tribasic calcium phosphate.
Lamotrigine tablets, USP comply with USP Dissolution Test 3.
Lamotrigine Tablets for Oral Suspension, USP comply with Organic Impurities Procedure 2.
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE | |||||
|
Phenylketonurics: What is the most important information I should know about Lamotrigine?
Call your healthcare provider right away if you have any of the following: *a skin rash *blistering or peeling of your skin *hives *painful sores in your mouth or around your eyes These symptoms may be the first signs of a serious skin reaction. A healthcare
provider should examine you to decide if you should continue taking
lamotrigine.
3. In patients with known heart problems, the use of lamotrigine may lead to a fast heart beat. Call your healthcare provider right away if you:
4. Like other antiepileptic drugs, lamotrigine may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
Do not stop lamotrigine without first talking to a healthcare provider.
How can I watch for early symptoms of suicidal thoughts and actions in myself or a family member?
5. Lamotrigine may cause aseptic meningitis, a serious inflammation of the protective membrane that covers the brain and spinal cord. Call your healthcare provider right away if you have any of the following symptoms:
Meningitis has many causes other than lamotrigine, which your doctor would
check for if you developed meningitis while taking lamotrigine.
These pictures show the distinct wording, colors, and shapes of the tablets
that help to identify the right strength of lamotrigine tablets and
lamotrigine tablets for oral suspension. Immediately call your pharmacist if
you receive a lamotrigine tablet that does not look like one of the tablets
shown below, as you may have received the wrong medication. | |||||
|
|
|
|
|
|
|
|
25 mg, white to off-white |
50 mg, white to off-white |
100 mg, white to off-white |
150 mg, white to off-white |
200 mg, white to off-white |
250 mg, white to-off white |
|
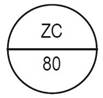
One side of the bisect is debossed with "ZC" and other side is debossed with "79" |
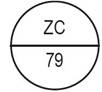
One side of the bisect is debossed with "ZC" and other side is debossed with "90" |
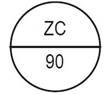
One side of the bisect is debossed with "ZC" and other side is debossed with "80" |
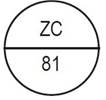
One side of the bisect is debossed with "ZC" and other side is debossed with "81" |
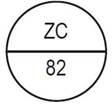
One side of the bisect is debossed with "ZC" and other side is debossed with "82" |
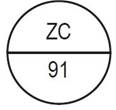
One side of the bisect is debossed with "ZC" and other side is debossed with "91" |
|
Lamotrigine Tablets for Oral Suspension | |||||
|
|
| ||||
|
5 mg, white to off-white |
25 mg, white to off-white | ||||
|
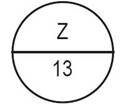
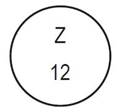
One side of the bisect is debossed with "Z" and other side |
Debossed with "Z" and "12" on one side | ||||
|
What is lamotrigine?
| |||||
|
Do not take lamotrigine:
| |||||
|
Before taking lamotrigine, tell your healthcare provider about all of your health conditions, including if you:
**Tell your healthcare provider about all the medicines you take,**including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Lamotrigine and certain other medicines may interact with each other. This may
cause serious side effects. | |||||
|
How should I take lamotrigine?
| |||||
|
What should I avoid while taking lamotrigine? | |||||
|
What are the possible side effects of lamotrigine? | |||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
| ||||
|
These are not all the possible side effects of lamotrigine. | |||||
|
How should I store lamotrigine?
| |||||
|
General information about the safe and effective use of lamotrigine | |||||
|
What are the ingredients in Lamotrigine? | |||||
|
Repackaged and Distributed By: Remedy Repack, Inc. 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762 | |||||
|
Rev.: 12/22 |
Repackaged By / Distributed By: RemedyRepack Inc.
625 Kolter Drive, Indiana, PA 15701
(724) 465-8762
DOSAGE FORMS & STRENGTHS SECTION
3. DOSAGE FORMS AND STRENGTHS
3.1 Tablets
200 mg, white to off-white, round, flat, beveled-edged tablets with bisect on one side; one side of bisect is debossed with logo of "ZC" and other side is debossed with "82" and other side is plain.
**Tablets:**25 mg, 50 mg, 100 mg, 150 mg, 200 mg, and 250 mg ( 3.1, 16)
***Tablets for Oral Suspension:**5 mg and 25 mg. ( 3.2, 16)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Epilepsy
Monotherapy with Lamotrigine in Adults with Partial-Onset Seizures Already Receiving Treatment with Carbamazepine, Phenytoin, Phenobarbital, or Primidone as the Single AED
The effectiveness of monotherapy with lamotrigine was established in a multicenter double-blind clinical trial enrolling 156 adult outpatients with partial-onset seizures. The patients experienced at least 4 simple partial- onset, complex partial-onset, and/or secondarily generalized seizures during each of 2 consecutive 4-week periods while receiving carbamazepine or phenytoin monotherapy during baseline. Lamotrigine (target dose of 500 mg/day) or valproate (1,000 mg/day) was added to either carbamazepine or phenytoin monotherapy over a 4-week period. Patients were then converted to monotherapy with lamotrigine or valproate during the next 4 weeks, then continued on monotherapy for an additional 12-week period.
Trial endpoints were completion of all weeks of trial treatment or meeting an escape criterion. Criteria for escape relative to baseline were: (1) doubling of average monthly seizure count, (2) doubling of highest consecutive 2-day seizure frequency, (3) emergence of a new seizure type (defined as a seizure that did not occur during the 8-week baseline) that is more severe than seizure types that occur during study treatment, or (4) clinically significant prolongation of generalized tonic-clonic (GTC) seizures. The primary efficacy variable was the proportion of patients in each treatment group who met escape criteria.
The percentages of patients who met escape criteria were 42% (32/76) in the group receiving lamotrigine and 69% (55/80) in the valproate group. The difference in the percentage of patients meeting escape criteria was statistically significant ( p=0.0012) in favor of lamotrigine. No differences in efficacy based on age, sex, or race were detected.
Patients in the control group were intentionally treated with a relatively low dose of valproate; as such, the sole objective of this trial was to demonstrate the effectiveness and safety of monotherapy with lamotrigine, and cannot be interpreted to imply the superiority of lamotrigine to an adequate dose of valproate.
Adjunctive Therapy with Lamotrigine in Adults with Partial-onset Seizures
The effectiveness of lamotrigine as adjunctive therapy (added to other AEDs) was initially established in 3 pivotal, multicenter, placebo-controlled, double-blind clinical trials in 355 adults with refractory partial-onset seizures. The patients had a history of at least 4 partial-onset seizures per month in spite of receiving 1 or more AEDs at therapeutic concentrations and in 2 of the trials were observed on their established AED regimen during baselines that varied between 8 to 12 weeks. In the third trial, patients were not observed in a prospective baseline. In patients continuing to have at least 4 seizures per month during the baseline, lamotrigine or placebo was then added to the existing therapy. In all 3 trials, change from baseline in seizure frequency was the primary measure of effectiveness. The results given below are for all partial-onset seizures in the intent-to-treat population (all patients who received at least 1 dose of treatment) in each trial, unless otherwise indicated. The median seizure frequency at baseline was 3 per week while the mean at baseline was 6.6 per week for all patients enrolled in efficacy trials.
One trial (n = 216) was a double-blind, placebo-controlled, parallel trial consisting of a 24-week treatment period. Patients could not be on more than 2 other anticonvulsants and valproate was not allowed. Patients were randomized to receive placebo, a target dose of 300 mg/day of lamotrigine, or a target dose of 500 mg/day of lamotrigine. The median reductions in the frequency of all partial-onset seizures relative to baseline were 8% in patients receiving placebo, 20% in patients receiving 300 mg/day of lamotrigine, and 36% in patients receiving 500 mg/day of lamotrigine. The seizure frequency reduction was statistically significant in the 500 mg/day group compared with the placebo group, but not in the 300 mg/day group.
A second trial (n = 98) was a double-blind, placebo-controlled, randomized, crossover trial consisting of two 14-week treatment periods (the last 2 weeks of which consisted of dose tapering) separated by a 4-week washout period. Patients could not be on more than 2 other anticonvulsants and valproate was not allowed. The target dose of lamotrigine was 400 mg/day. When the first 12 weeks of the treatment periods were analyzed, the median change in seizure frequency was a 25% reduction on lamotrigine compared with placebo ( p<0.001).
The third trial (n = 41) was a double-blind, placebo-controlled, crossover trial consisting of two 12-week treatment periods separated by a 4-week washout period. Patients could not be on more than 2 other anticonvulsants. Thirteen patients were on concomitant valproate; these patients received 150 mg/day of lamotrigine. The 28 other patients had a target dose of 300 mg/day of lamotrigine. The median change in seizure frequency was a 26% reduction on lamotrigine compared with placebo ( p<0.01).
No differences in efficacy based on age, sex, or race, as measured by change in seizure frequency, were detected.
Adjunctive Therapy with Lamotrigine in Pediatric Patients with Partial-Onset Seizures
The effectiveness of lamotrigine as adjunctive therapy in pediatric patients with partial-onset seizures was established in a multicenter, double-blind, placebo-controlled trial in 199 patients aged 2 to 16 years (n = 98 on lamotrigine, n = 101 on placebo). Following an 8-week baseline phase, patients were randomized to 18 weeks of treatment with lamotrigine or placebo added to their current AED regimen of up to 2 drugs. Patients were dosed based on body weight and valproate use. Target doses were designed to approximate 5 mg/kg/day for patients taking valproate (maximum dose: 250 mg/day) and 15 mg/kg/day for the patients not taking valproate (maximum dose: 750 mg/day). The primary efficacy endpoint was percentage change from baseline in all partial-onset seizures. For the intent-to-treat population, the median reduction of all partial-onset seizures was 36% in patients treated with lamotrigine and 7% on placebo, a difference that was statistically significant ( p<0.01).
Adjunctive Therapy with Lamotrigine in Pediatric and Adult Patients with Lennox-Gastaut Syndrome
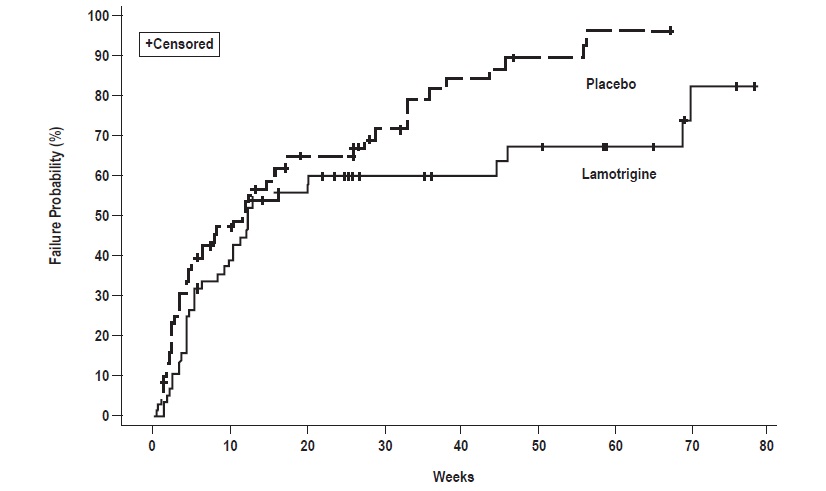
The effectiveness of lamotrigine as adjunctive therapy in patients with Lennox-Gastaut syndrome was established in a multicenter, double-blind, placebo-controlled trial in 169 patients aged 3 to 25 years (n = 79 on lamotrigine, n = 90 on placebo). Following a 4-week, single-blind, placebo phase, patients were randomized to 16 weeks of treatment with lamotrigine or placebo added to their current AED regimen of up to 3 drugs. Patients were dosed on a fixed-dose regimen based on body weight and valproate use. Target doses were designed to approximate 5 mg/kg/day for patients taking valproate (maximum dose: 200 mg/day) and 15 mg/kg/day for patients not taking valproate (maximum dose: 400 mg/day). The primary efficacy endpoint was percentage change from baseline in major motor seizures (atonic, tonic, major myoclonic, and tonic-clonic seizures). For the intent-to-treat population, the median reduction of major motor seizures was 32% in patients treated with lamotrigine and 9% on placebo, a difference that was statistically significant ( p<0.05). Drop attacks were significantly reduced by lamotrigine (34%) compared with placebo (9%), as were tonic-clonic seizures (36% reduction versus 10% increase for lamotrigine and placebo, respectively).
Adjunctive Therapy with Lamotrigine in Pediatric and Adult Patients with Primary Generalized Tonic-Clonic Seizures****
The effectiveness of lamotrigine as adjunctive therapy in patients with PGTC seizures was established in a multicenter, double-blind, placebo-controlled trial in 117 pediatric and adult patients aged 2 years and older (n = 58 on lamotrigine, n = 59 on placebo). Patients with at least 3 PGTC seizures during an 8-week baseline phase were randomized to 19 to 24 weeks of treatment with lamotrigine or placebo added to their current AED regimen of up to 2 drugs. Patients were dosed on a fixed-dose regimen, with target doses ranging from 3 to 12 mg/kg/day for pediatric patients and from 200 to 400 mg/day for adult patients based on concomitant AEDs.
The primary efficacy endpoint was percentage change from baseline in PGTC seizures. For the intent-to-treat population, the median percent reduction in PGTC seizures was 66% in patients treated with lamotrgine and 34% on placebo, a difference that was statistically significant ( p= 0.006).
14.2 Bipolar Disorder
Adults
The effectiveness of lamotrigine in the maintenance treatment of bipolar I disorder was established in 2 multicenter, double-blind, placebo-controlled trials in adult patients (aged 18 to 82 years) who met DSM-IV criteria for bipolar I disorder. Trial 1 enrolled patients with a current or recent (within 60 days) depressive episode as defined by DSM-IV and Trial 2 included patients with a current or recent (within 60 days) episode of mania or hypomania as defined by DSM-IV. Both trials included a cohort of patients (30% of 404 subjects in Trial 1 and 28% of 171 patients in Trial 2) with rapid cycling bipolar disorder (4 to 6 episodes per year).
In both trials, patients were titrated to a target dose of 200 mg of lamotrigine as add-on therapy or as monotherapy with gradual withdrawal of any psychotropic medications during an 8- to 16-week open-label period. Overall 81% of 1,305 patients participating in the open-label period were receiving 1 or more other psychotropic medications, including benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), atypical antipsychotics (including olanzapine), valproate, or lithium, during titration of lamotrigine. Patients with a CGI-severity score of 3 or less maintained for at least 4 continuous weeks, including at least the final week on monotherapy with lamotrigine, were randomized to a placebo-controlled double-blind treatment period for up to 18 months. The primary endpoint was TIME (time to intervention for a mood episode or one that was emerging, time to discontinuation for either an adverse event that was judged to be related to bipolar disorder, or for lack of efficacy). The mood episode could be depression, mania, hypomania, or a mixed episode.
In Trial 1, patients received double-blind monotherapy with lamotrigine 50 mg/day (n = 50), lamotrigine 200 mg/day (n = 124), lamotrigine 400 mg/day (n = 47), or placebo (n = 121). Lamotrigine (200 and 400 mg/day treatment groups combined) was superior to placebo in delaying the time to occurrence of a mood episode (Figure 1). Separate analyses of the 200 and 400 mg/day dose groups revealed no added benefit from the higher dose.
In Trial 2, patients received double-blind monotherapy with lamotrigine (100 to 400 mg/day, n = 59), or placebo (n = 70). Lamotrigine was superior to placebo in delaying time to occurrence of a mood episode (Figure 2). The mean dose of lamotrigine was about 211 mg/day.
Although these trials were not designed to separately evaluate time to the occurrence of depression or mania, a combined analysis for the 2 trials revealed a statistically significant benefit for lamotrigine over placebo in delaying the time to occurrence of both depression and mania, although the finding was more robust for depression.
Figure 1: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Mood Episode (Trial 1)
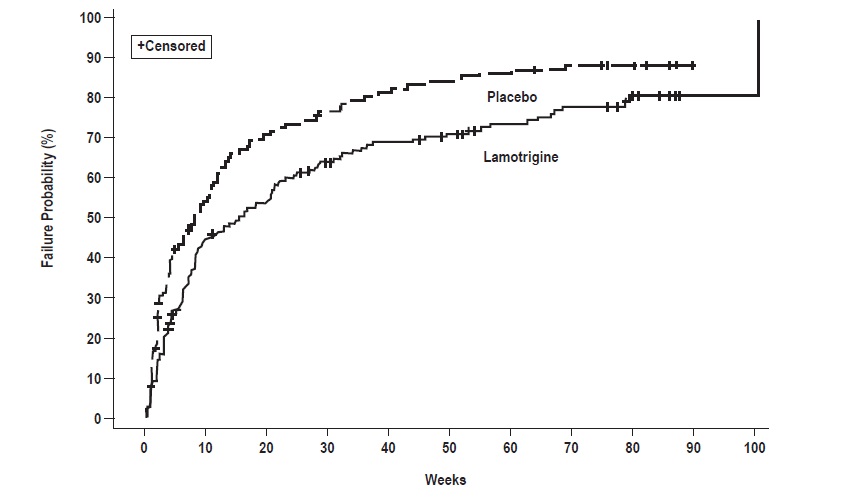
Figure 2: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Mood Episode (Trial 2)