Finasteride
These highlights do not include all the information needed to use FINASTERIDE TABLETS safely and effectively. See full prescribing information for FINASTERIDE TABLETS. FINASTERIDE tablets, for oral use Initial U.S. Approval: 1992
8f468081-254a-4f11-9f0b-c0311e353a50
HUMAN PRESCRIPTION DRUG LABEL
Apr 26, 2023
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Finasteride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 5 mg

Finasteride
Tablet, USP
5 mg
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Prostate Specific Antigen (PSA) and the Use of PSA in
Prostate Cancer Detection
In clinical studies, finasteride reduced serum PSA concentration by approximately 50% within six months of treatment. This decrease is predictable over the entire range of PSA values in patients with symptomatic BPH, although it may vary in individuals.
For interpretation of serial PSAs in men taking finasteride, a new PSA baseline should be established at least six months after starting treatment and PSA monitored periodically thereafter. Any confirmed increase from the lowest PSA value while on finasteride may signal the presence of prostate cancer and should be evaluated, even if PSA levels are still within the normal range for men not taking a 5α‑reductase inhibitor. Non-compliance with finasteride therapy may also affect PSA test results. To interpret an isolated PSA value in patients treated with finasteride for six months or more, PSA values should be doubled for comparison with normal ranges in untreated men. These adjustments preserve the utility of PSA to detect prostate cancer in men treated with finasteride.
Finasteride may also cause decreases in serum PSA in the presence of prostate cancer.
The ratio of free to total PSA (percent free PSA) remains constant even under the influence of finasteride. If clinicians elect to use percent free PSA as an aid in the detection of prostate cancer in men undergoing finasteride therapy, no adjustment to its value appears necessary.
5.2 Increased Risk of High-Grade Prostate Cancer
Men aged 55 and over with a normal digital rectal examination and PSA ≤3 ng/mL at baseline taking finasteride 5 mg/day in the 7-year Prostate Cancer Prevention Trial (PCPT) had an increased risk of Gleason score 8 to 10 prostate cancer (finasteride 1.8% vs. placebo 1.1%). [See Indications and Usage (1.3) and Adverse Reactions (6.1).] Similar results were observed in a 4-year placebo-controlled clinical trial with another 5α-reductase inhibitor (dutasteride, AVODART) (1% dutasteride vs. 0.5% placebo). 5α-reductase inhibitors may increase the risk of development of high-grade prostate cancer. Whether the effect of 5α-reductase inhibitors to reduce prostate volume, or study-related factors, impacted the results of these studies has not been established.
5.3 Exposure of Females - Risk to Male Fetus
Finasteride is contraindicated in pregnant females and in females who may potentially be pregnant and not indicated for use in females. Based on animal studies and the mechanism of action, finasteride may cause abnormal development of external genitalia in a male fetus if administered to a pregnant female. Females who are pregnant or may potentially be pregnant should not handle crushed or broken finasteride tablets. Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. If a pregnant female comes in contact with crushed or broken finasteride tablets, the contact area should be washed immediately with soap and water. [See Contraindications (4), Use in Specific Populations (8.1), Clinical Pharmacology (12.1 and 12.3), How Supplied/Storage and Handling (16)].
5.4 Pediatric Patients and Females
Finasteride is not indicated for use in pediatric patients [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)] or females [see also Warnings and Precautions (5.3), Use in Specific Populations (8.1), Clinical Pharmacology (12.3), How Supplied/Storage and Handling (16)].
5.5 Effect on Semen Characteristics
Treatment with finasteride for 24 weeks to evaluate semen parameters in healthy male volunteers revealed no clinically meaningful effects on sperm concentration, mobility, morphology, or pH. A 0.6 mL (22.1%) median decrease in ejaculate volume with a concomitant reduction in total sperm per ejaculate was observed. These parameters remained within the normal range and were reversible upon discontinuation of therapy with an average time to return to baseline of 84 weeks.
5.6 Consideration of Other Urological Conditions
Prior to initiating treatment with finasteride, consideration should be given to other urological conditions that may cause similar symptoms. In addition, prostate cancer and BPH may coexist.
Patients with large residual urinary volume and/or severely diminished urinary flow should be carefully monitored for obstructive uropathy. These patients may not be candidates for finasteride therapy.
- Finasteride reduces serum prostate specific antigen (PSA) levels by approximately 50%. However, any confirmed increase in PSA while on finasteride may signal the presence of prostate cancer and should be evaluated, even if those values are still within the normal range for men not taking a 5α-reductase inhibitor ( 5.1).
- Finasteride may increase the risk of high-grade prostate cancer ( 5.2, 6.1).
- Females should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant due to potential risk to a male fetus ( 5.3, 8.1, 16).
- Finasteride is not indicated for use in pediatric patients or females ( 5.4, 8.1, 8.3, 8.4, 12.3).
- Prior to initiating treatment with finasteride for BPH, consideration should be given to other urological conditions that may cause similar symptoms ( 5.6).
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System
No drug interactions of clinical importance have been identified. Finasteride does not appear to affect the cytochrome P450-linked drug metabolizing enzyme system. Compounds that have been tested in man have included antipyrine, digoxin, propranolol, theophylline, and warfarin and no clinically meaningful interactions were found.
7.2 Other Concomitant Therapy
Although specific interaction studies were not performed, finasteride was concomitantly used in clinical studies with acetaminophen, acetylsalicylic acid, α-blockers, angiotensin-converting enzyme (ACE) inhibitors, analgesics, anti-convulsants, beta-adrenergic blocking agents, diuretics, calcium channel blockers, cardiac nitrates, HMG-CoA reductase inhibitors, nonsteroidal anti- inflammatory drugs (NSAIDs), benzodiazepines, H 2 antagonists and quinolone anti-infectives without evidence of clinically significant adverse interactions.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Monotherapy
Finasteride 5 mg/day was initially evaluated in patients with symptoms of BPH and enlarged prostates by digital rectal examination in two 1-year, placebo- controlled, randomized, double-blind studies and their 5-year open extensions.
Finasteride was further evaluated in a long-term efficacy and safety study, a double-blind, randomized, placebo-controlled, 4-year, multicenter study. 3040 patients between the ages of 45 and 78, with moderate to severe symptoms of BPH and an enlarged prostate upon digital rectal examination, were randomized into the study (1524 to finasteride, 1516 to placebo) and 3016 patients were evaluable for efficacy. 1883 patients completed the 4-year study (1000 in the finasteride group, 883 in the placebo group).
Effect on Symptom Score
Symptoms were quantified using a score similar to the American Urological
Association Symptom Score, which evaluated both obstructive symptoms
(impairment of size and force of stream, sensation of incomplete bladder
emptying, delayed or interrupted urination) and irritative symptoms (nocturia,
daytime frequency, need to strain or push the flow of urine) by rating on a 0
to 5 scale for six symptoms and a 0 to 4 scale for one symptom, for a total
possible score of 34.
Patients in a long-term efficacy and safety study had moderate to severe symptoms at baseline (mean of approximately 15 points on a 0 to 34 point scale). Patients randomized to finasteride who remained on therapy for 4 years had a mean (± 1 SD) decrease in symptom score of 3.3 (± 5.8) points compared with 1.3 (± 5.6) points in the placebo group. (See Figure 1.) A statistically significant improvement in symptom score was evident at 1 year in patients treated with finasteride vs. placebo (–2.3 vs. –1.6), and this improvement continued through Year 4.
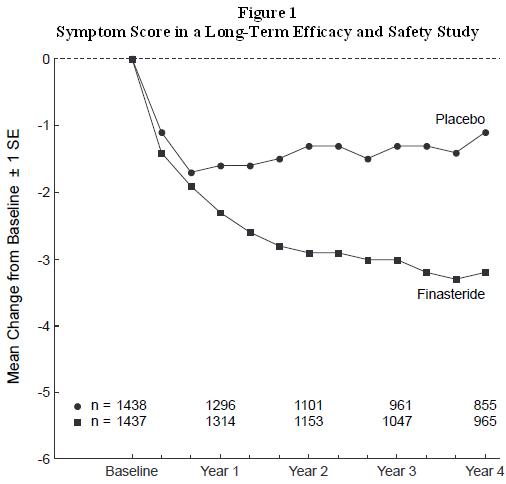
Results seen in earlier studies were comparable to those seen in a long-term efficacy and safety study. Although an early improvement in urinary symptoms was seen in some patients, a therapeutic trial of at least 6 months was generally necessary to assess whether a beneficial response in symptom relief had been achieved. The improvement in BPH symptoms was seen during the first year and maintained throughout an additional 5 years of open extension studies.
Effect on Acute Urinary Retention and the Need for Surgery
In a long-term efficacy and safety study, efficacy was also assessed by
evaluating treatment failures. Treatment failure was prospectively defined as
BPH-related urological events or clinical deterioration, lack of improvement
and/or the need for alternative therapy. BPH-related urological events were
defined as urological surgical intervention and acute urinary retention
requiring catheterization. Complete event information was available for 92% of
the patients. The following table (Table 5) summarizes the results.
| |||||
|
Patients (%)* | |||||
|
Event |
Placebo N=1503 |
Finasteride N=1513 |
Relative Risk† |
95% CI |
P Value† |
|
All Treatment Failures |
37.1 |
26.2 |
0.68 |
(0.57 to 0.79) |
<0.001 |
|
Surgical Interventions for BPH |
10.1 |
4.6 |
0.45 |
(0.32 to 0.63) |
<0.001 |
|
Acute Urinary Retention Requiring Catheterization |
6.6 |
2.8 |
0.43 |
(0.28 to 0.66) |
<0.001 |
|
Two consecutive symptom scores ≥20 |
9.2 |
6.7 | |||
|
Bladder Stone |
0.4 |
0.5 | |||
|
Incontinence |
2.1 |
1.7 | |||
|
Renal Failure |
0.5 |
0.6 | |||
|
UTI |
5.7 |
4.9 | |||
|
Discontinuation due to worsening of BPH, lack of improvement, or to receive other medical treatment |
21.8 |
13.3 |
Compared with placebo, finasteride was associated with a significantly lower risk for acute urinary retention or the need for BPH-related surgery [13.2% for placebo vs 6.6% for finasteride; 51% reduction in risk, 95% CI: (34 to 63%)]. Compared with placebo, finasteride was associated with a significantly lower risk for surgery [10.1% for placebo vs 4.6% for finasteride; 55% reduction in risk, 95% CI: (37 to 68%)] and with a significantly lower risk of acute urinary retention [6.6% for placebo vs 2.8% for finasteride; 57% reduction in risk, 95% CI: (34 to 72%)]; see Figures 2 and 3.
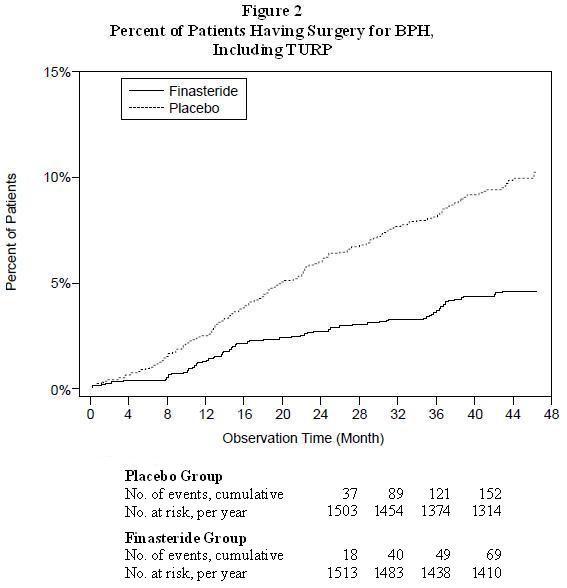
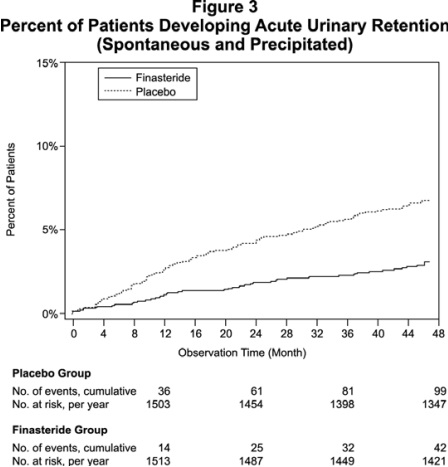
Effect on Maximum Urinary Flow Rate
In the patients in a long-term efficacy and safety study who remained on
therapy for the duration of the study and had evaluable urinary flow data,
finasteride increased maximum urinary flow rate by 1.9 mL/sec compared with
0.2 mL/sec in the placebo group.
There was a clear difference between treatment groups in maximum urinary flow rate in favor of finasteride by month 4 (1 vs. 0.3 mL/sec) which was maintained throughout the study. In the earlier 1-year studies, increase in maximum urinary flow rate was comparable to a long-term efficacy and safety study and was maintained through the first year and throughout an additional 5 years of open extension studies.
Effect on Prostate Volume
In a long-term efficacy and safety study, prostate volume was assessed yearly
by magnetic resonance imaging (MRI) in a subset of patients. In patients
treated with finasteride who remained on therapy, prostate volume was reduced
compared with both baseline and placebo throughout the 4-year study.
Finasteride decreased prostate volume by 17.9% (from 55.9 cc at baseline to
45.8 cc at 4 years) compared with an increase of 14.1% (from 51.3 cc to 58.5
cc) in the placebo group (p<0.001). (See Figure 4.)
Results seen in earlier studies were comparable to those seen in a long-term efficacy and safety study. Mean prostate volume at baseline ranged between 40 to 50 cc. The reduction in prostate volume was seen during the first year and maintained throughout an additional five years of open extension studies.
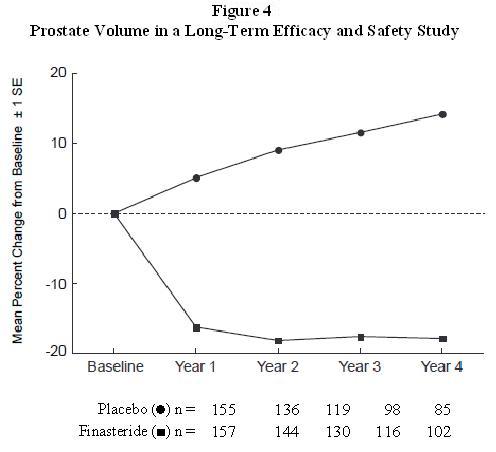
Prostate Volume as a Predictor of Therapeutic Response
A meta-analysis combining 1-year data from seven double-blind, placebo-
controlled studies of similar design, including 4491 patients with symptomatic
BPH, demonstrated that, in patients treated with finasteride, the magnitude of
symptom response and degree of improvement in maximum urinary flow rate were
greater in patients with an enlarged prostate at baseline.
14.2 Combination with Alpha-Blocker Therapy
The Medical Therapy of Prostatic Symptoms (MTOPS) Trial was a double-blind, randomized, placebo-controlled, multicenter, 4- to 6-year study (average 5 years) in 3047 men with symptomatic BPH, who were randomized to receive finasteride 5 mg/day (n=768), doxazosin 4 or 8 mg/day (n=756), the combination of finasteride 5 mg/day and doxazosin 4 or 8 mg/day (n=786), or placebo (n=737). All participants underwent weekly titration of doxazosin (or its placebo) from 1 to 2 to 4 to 8 mg/day. Only those who tolerated the 4 or 8 mg dose level were kept on doxazosin (or its placebo) in the study. The participant’s final tolerated dose (either 4 mg or 8 mg) was administered beginning at end-Week 4. The final doxazosin dose was administered once per day, at bedtime.
The mean patient age at randomization was 62.6 years (±7.3 years). Patients were Caucasian (82%), African American (9%), Hispanic (7%), Asian (1%) or Native American (<1%). The mean duration of BPH symptoms was 4.7 years (±4.6 years). Patients had moderate to severe BPH symptoms at baseline with a mean AUA symptom score of approximately 17 out of 35 points. Mean maximum urinary flow rate was 10.5 mL/sec (±2.6 mL/sec). The mean prostate volume as measured by transrectal ultrasound was 36.3 mL (±20.1 mL). Prostate volume was ≤20 mL in 16% of patients, ≥50 mL in 18% of patients and between 21 and 49 mL in 66% of patients.
The primary endpoint was a composite measure of the first occurrence of any of the following five outcomes: a ≥4 point confirmed increase from baseline in symptom score, acute urinary retention, BPH-related renal insufficiency (creatinine rise), recurrent urinary tract infections or urosepsis, or incontinence. Compared to placebo, treatment with finasteride, doxazosin, or combination therapy resulted in a reduction in the risk of experiencing one of these five outcome events by 34% (p=0.002), 39% (p<0.001), and 67% (p<0.001), respectively. Combination therapy resulted in a significant reduction in the risk of the primary endpoint compared to treatment with finasteride alone (49%; p≤0.001) or doxazosin alone (46%; p≤0.001). (See Table 6.)
Table 6: Count and Percent Incidence of Primary Outcome Events by Treatment Group in MTOPS|
Event |
Treatment Group | ||||
|
Placebo N=737 N (%) |
Doxazosin N=756 N (%) |
Finasteride N=768 N (%) |
Combination N=786 N (%) |
Total N=3047 N (%) | |
|
AUA 4-point rise |
100 (13.6) |
59 (7.8) |
74 (9.6) |
41 (5.2) |
274 (9) |
|
Acute urinary retention |
18 (2.4) |
13 (1.7) |
6 (0.8) |
4 (0.5) |
41 (1.3) |
|
Incontinence |
8 (1.1) |
11 (1.5) |
9 (1.2) |
3 (0.4) |
31 (1) |
|
Recurrent UTI/urosepsis |
2 (0.3) |
2 (0.3) |
0 (0) |
1 (0.1) |
5 (0.2) |
|
Creatinine rise |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
Total Events |
110 (15) |
72 (9.6) |
83 (10.8) |
45 (5.7) |
310 (10.2) |
The majority of the events (274 out of 351; 78%) was a confirmed ≥4 point increase in symptom score, referred to as symptom score progression. The risk of symptom score progression was reduced by 30% (p=0.016), 46% (p<0.001), and 64% (p<0.001) in patients treated with finasteride, doxazosin, or the combination, respectively, compared to patients treated with placebo (see Figure 5). Combination therapy significantly reduced the risk of symptom score progression compared to the effect of finasteride alone (p<0.001) and compared to doxazosin alone (p=0.037).
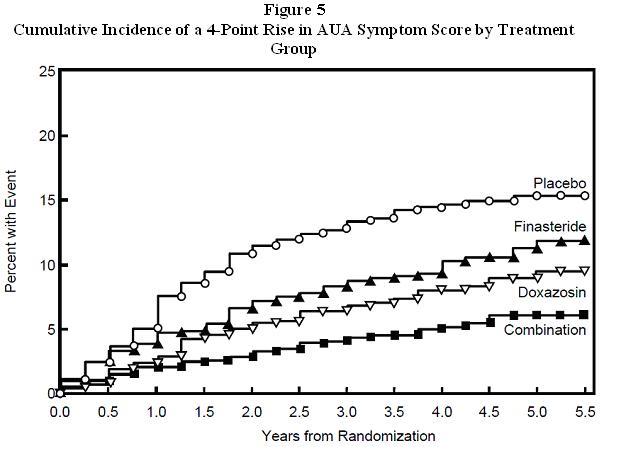
Treatment with finasteride, doxazosin or the combination of finasteride with doxazosin, reduced the mean symptom score from baseline at year 4. Table 7 provides the mean change from baseline for AUA symptom score by treatment group for patients who remained on therapy for four years.
Table 7: Change From Baseline in AUA Symptom Score by Treatment Group at Year 4 in MTOPS|
Placebo |
Doxazosin |
Finasteride |
Combination | |
|---|---|---|---|---|
|
Baseline Mean (SD) |
16.8 (6) |
17 (5.9) |
17.1 (6) |
16.8 (5.8) |
|
Mean Change AUA Symptom Score (SD) |
-4.9 (5.8) |
-6.6 (6.1) |
-5.6 (5.9) |
-7.4 (6.3) |
|
Comparison to Placebo (95% CI) |
-1.8 |
-0.7 |
-2.5 | |
|
Comparison to Doxazosin alone (95% CI) |
-0.7 | |||
|
Comparison to Finasteride alone (95% CI) |
-1.8 |
The results of MTOPS are consistent with the findings of the 4-year, placebo- controlled study a long-term efficacy and safety study [see Clinical Studies (14.1)] in that treatment with finasteride reduces the risk of acute urinary retention and the need for BPH-related surgery. In MTOPS, the risk of developing acute urinary retention was reduced by 67% in patients treated with finasteride compared to patients treated with placebo (0.8% for finasteride and 2.4% for placebo). Also, the risk of requiring BPH-related invasive therapy was reduced by 64% in patients treated with finasteride compared to patients treated with placebo (2% for finasteride and 5.4% for placebo).
14.3 Summary of Clinical Studies
The data from these studies, showing improvement in BPH-related symptoms, reduction in treatment failure (BPH-related urological events), increased maximum urinary flow rates, and decreasing prostate volume, suggest that finasteride arrests the disease process of BPH in men with an enlarged prostate.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling ( Patient Information).
17.1 Increased Risk of High-Grade Prostate Cancer
Patients should be informed that there was an increase in high-grade prostate cancer in men treated with 5α-reductase inhibitors indicated for BPH treatment, including finasteride, compared to those treated with placebo in studies looking at the use of these drugs to prevent prostate cancer [see Indications and Usage (1.3), Warnings and Precautions (5.2), and Adverse Reactions (6.1)].
17.2 Exposure of Females - Risk to Male Fetus
Physicians should inform patients that females who are pregnant or may potentially be pregnant should not handle crushed or broken finasteride tablets because of the possibility of absorption of finasteride and the subsequent potential risk to the male fetus. Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. If a female who is pregnant or may potentially be pregnant comes in contact with crushed or broken finasteride tablets, the contact area should be washed immediately with soap and water [see Contraindications (4), Warnings and Precautions (5.3), Use in Specific Populations (8.1) and How Supplied/Storage and Handling (16)].
17.3 Additional Instructions
Physicians should inform patients that the volume of ejaculate may be decreased in some patients during treatment with finasteride. This decrease does not appear to interfere with normal sexual function. However, impotence and decreased libido may occur in patients treated with finasteride [see Adverse Reactions (6.1)].
Physicians should instruct their patients to promptly report any changes in their breasts such as lumps, pain or nipple discharge. Breast changes including breast enlargement, tenderness and neoplasm have been reported [see Adverse Reactions (6.1)].
Physicians should instruct their patients to read the patient package insert before starting therapy with finasteride and to reread it each time the prescription is renewed so that they are aware of current information for patients regarding finasteride.
